Alkynes: Properties, Preparation & Reactions - Chemistry Notes
advertisement

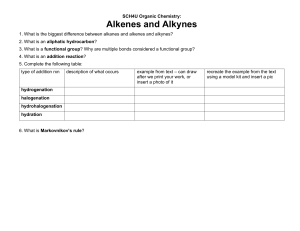
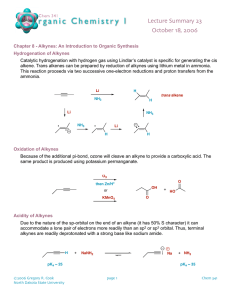
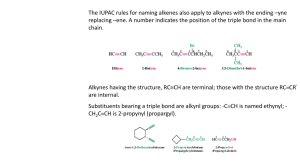
ALKYNES • Hydrocarbons containing a carbon-carbon triple bond are called alkynes. • The simplest alkyne is acetylene (H-C = C-H as by its common name. The names of alkynes generally follow the IUPAC system. • The longest chain containing the C = C bond is selected as the base structure. It’s name is derived from the analogous alkane but ‘yne’ replaces ‘ane’. • The carbons are numbered from the end nearest to the C = C bond. • The general formula is Cn H . 2 n 2 3. Hydrocarbon, halogen and other groups are named as substituents with the number of the carbon to which the substituent is attached. INDUSTRIAL USES OF ALKYNES • (i) Large quantities of ethyne are consumed every year. Dissolved under pressure in acetone, it is sold to be used as fuel in oxyacetylene torch. • (ii)It is the organic starting material for the large scale synthesis of important organic compounds e.g. CH3 C O O H . PREPARATION OF ALKYNES A carbon-carbon triple bond is formed in the same way as a double bond, i.e. elimination of atoms or groups from two adjacent carbons. Examples: (a) Dehydrohalogenation of alkyl dihalides The dihalides are readily obtained from corresponding alkenes by addition of halogen. This amounts to conversion – by several steps – of a double bond into a triple bond. INDUSTRIAL SOURCES OF Ethyne is obtainedALKYNES in large quantities from coke, the solid residue from the distillation of volatile compounds of coal. Coke is heated with calcium oxide CaO at 2000 oC to give calcium carbide and carbon monoxide. Calcium carbide and water react to give Ethyne and CaO. (b) Reaction of Sodium acetylides with primary alkyl halides This permits the conversion of smaller alkynes into larger ones. Practically, the reaction is limited to the use of primary halides because of the great tendency for secondary and tertiary halides to undergo a side reaction. (c) Dehalogenation of Tetrahalides REACTION OF ALKYNES • Just like the alkenes, the chemistry of alkynes is the chemistry of the carbon carbon triple bond. Like alkenes, alkynes undergo electrophilic addition. • The same reagents will add to triple bonds as to the double bonds with some variations in conditions. The reactions of alkynes can usually be stopped after the first stage of addition to give alkene products. (a) Catalytic Hydrogenation Catalysts for hydrogenation are same metals as for alkene, Pt or Pd. The two hydrogens add from the same side of the triple bond to give Z alkene, whereas with, Na, NH3 ( l i q ) t h e p r o d u c t i s m a i n l y ( E ) . e.g. (b) Addition of Hydrogen Halide The direction of the addition of HCl to alkyne follows the Markovnikov’s rule. The carbocation intermediate formed is the more stable one i.e. (c) Addition of Water Hydration of alkynes requires catalysis by both H2 S O a n d 4 2 + m e r c u r i c s u l p h a t e . M e r c u r i c i o n ( H g ) i s k n o w n t o f o r m a n a s s o c i a t i o n w i t h a n a l k y n e w h e r e b y t h e t r i p l e b o n d i s w e a k e n e d . The addition gives first an unstable enol which quickly rearranges to a more stable aldehyde or ketone. The addition of water follows the Markovnikov’s rule. e.g. (d) Addition of Halogens Bromine and chlorine add to alkynes to form dihaloalkenes and tetra haloalkanes ACIDITY OF ALKYNES However, disubstituted alkynes which have no replaceable hydrogen atom do not form such salts. Sodium reacts with ethylene to liberate hydrogen gas. i.e. Sodium acetylides are often used in the synthesis of higher alkynes SHORT QUIZ ON CHEMISTRY OF ALKYNES