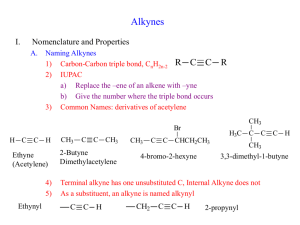
Module No. 03(Q2) Structure and nomenclature of Hydrocarbon compounds Describe alkyne as hydrocarbon compound. Specific Objectives At the end of this module, learners are expected to: 1. describe and name simple alkynes; 2. use the general formula for alkyne to predict its molecular formula and structural and condensed formula; and 3. draw the structures of alkyne. Materials • • • Pen Bond paper or intermediate paper Any Chemistry book or Laboratory Manual Lesson Another aliphatic hydrocarbon is alkyne which is under unsaturated hydrocarbon. How are we going to distinguish alkane from alkene and alkyne? Let’s start the discussion to compare at the later part. 1 The alkynes (also called acetylene) contain at least one carboncarbon triple bond. It has a general formula of CnH2n -2, where n = 2, 3, . . . . The simplest alkyne is C2H2, ethylene, which is use for cutting and welding metals. Examples of Alkynes Naming Alkynes In naming alkynes, we follow the same guidelines used in alkanes except that we first locate the presence of triple bond and assign the lowest possible number in its location. The names of compounds containing triple bonds end with -yne. As with the alkanes, the name of the parent compound is determined by the number of carbon atoms in the longest chain. Numbering the parent chain begins at the end nearer the triple bond so that the triple 2 bond receives a lower number. Compounds with more than one triple bond are called diynes, triynes, and so forth. Give the IUPAC names of the following compounds: https://www.google.com/search?q=alkynes+examples&rlz=1C1SQJL_enPH774PH774&sxsrf=ALeKk025vY6LjIT9chG91AfJKB_Cc5wWw:1597895018619&tbm=isch&source=iu&ictx=1&fir=cql08v4UKETRCM%252C14c0C4njNqxdwM%252C_&vet=1&usg=AI4_kR8qUKguXvBDvV37o1CHhduhNaYNA&sa=X&ved=2ahUKEwi7kOTn7qjrAhUBfd4KHS57A1QQ_h0wA3oECAoQCg&biw=1366&bih=657#imgrc=57Y_B hhh7T4s7M If you will name the two compounds, the first one is 2,2-dimethyl-3hexyne and the second one is 5-methyl-2-hexyne. Directions: Do what is asked in the following given. 1. Draw the condensed formula for the following: a. 3-methyl-1-butyne b. 2,5,6-trimethyl-3,3-heptyne c. 3-ethyl-4,5,5-trimethyl-1-hexyne d. 2,5-dimethyl-3-heptyne e. 4,4,5-trimethyl-2-hexyne Agreement: Give the IUPAC name of the following: 1. 2. 3 3. 4. References Chang, Raymond and Jayson Overby…General Chemistry The Essential Concepts 6Th Edition. New York: The McGraw Hill Companies Inc, 2011 Key to Correction 1. 2. 4 3. 4. 5. 5