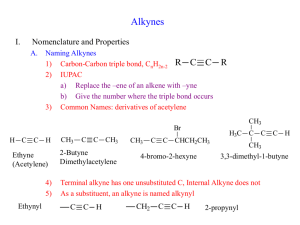
Naming the Alkynes
advertisement
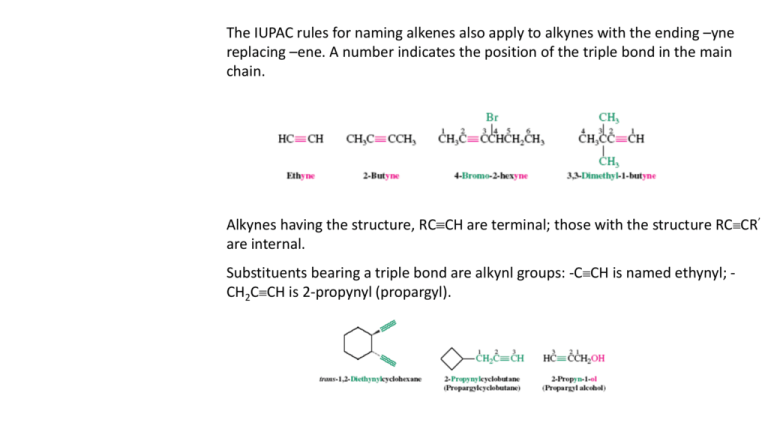
The IUPAC rules for naming alkenes also apply to alkynes with the ending –yne replacing –ene. A number indicates the position of the triple bond in the main chain. Alkynes having the structure, RCCH are terminal; those with the structure RCCR’ are internal. Substituents bearing a triple bond are alkynl groups: -CCH is named ethynyl; CH2CCH is 2-propynyl (propargyl). In IUPAC nomenclature, a hydrocarbon containing both a double and a triple bond is called an alkenyne. The chain is numbered starting at the end closest to either functional group. In the case of a tie, the double bond is given the lower number. Alkynes containing a hydroxyl group are named alkynols. In this case, the –OH takes precedence over both double and triple bonds in numbering the chain. 13-2 Properties and Bonding in the Alkynes Alkynes are relatively nonpolar. Corresponding alkynes, alkenes and alkanes have very similar boiling points. Ethyne: sublimes at -84oC Propyne: b.p. -23.2oC 1-Butyne: b.p. 8.1oC 2-Butyne: b.p. 27oC Medium sized alkanes: distillable liquids Alkynes polymerize easily, frequently with violence. Ethyne is linear and has strong, short bonds. The two carbons in ethyne are sp2 hybridized. The bonds are diffuse and resemble a cylindrical cloud: The strength of a C-C triple bond is about 229 kcal mol-1. As with alkenes, the strength of the bonds is much weaker than that of the δ bond which gives rise to much of the chemical reactivity of the alkynes. The CC bond length is 1.20 Å (C=C is 1.33 Å). The C-H bond lengths are also shorter than in ethene due to the larger degree of s character in the sp hybrid bonds (as compared to sp2). Alkynes are high-energy compounds. Alkynes often react with the release of considerable amounts of energy (prone to explosive decomposition). Ethyne has a heat of combustion of 311 kcal mol-1 which is capable of generating a flame temperature >2500o C, sufficient for use in welding torches. The heats of hydrogenation of alkyne isomers can be used to determine their relative stabilities: The greater relative stability of internal alkynes is due to hyperconjugation. Terminal alkynes are remarkably acidic. The electronegativity of a carbon atom depend upon its hybridization. The more s character in its hybrid orbitals, the greater the electronegativity. The acidity of a C-H bond is directly related to the electronegativity of the carbon atom: Strong bases such as sodium amide in liquid ammonia, alkyllithiums, and Grignard reagents can deprotonate terminal alkynes to the corresponding alkynyl anions. Alkynyl anions can react as bases and nucleophiles, much like other carbanions.