Chapter 5 Stereoisomers I. Introduction A.
advertisement
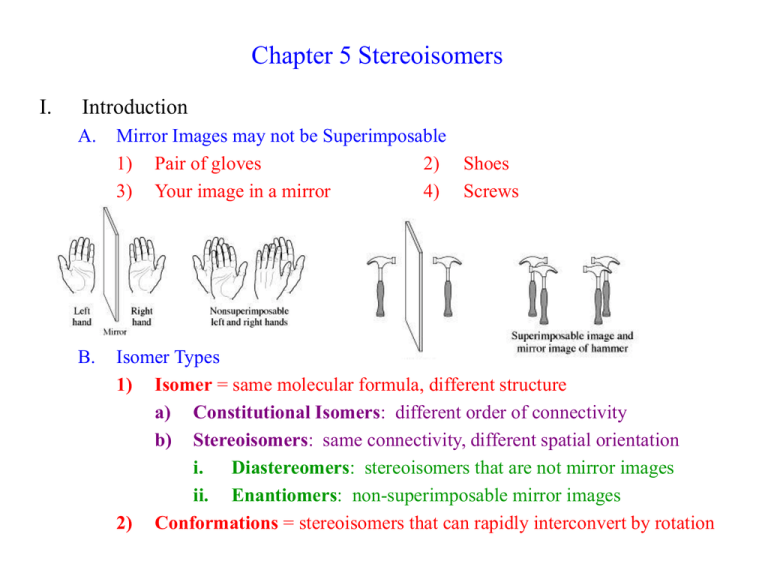
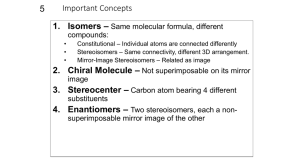
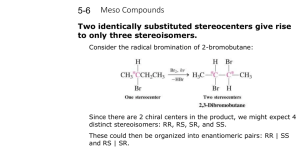
Chapter 5 Stereoisomers I. Introduction A. Mirror Images may not be Superimposable 1) Pair of gloves 2) Shoes 3) Your image in a mirror 4) Screws B. Isomer Types 1) Isomer = same molecular formula, different structure a) Constitutional Isomers: different order of connectivity b) Stereoisomers: same connectivity, different spatial orientation i. Diastereomers: stereoisomers that are not mirror images ii. Enantiomers: non-superimposable mirror images 2) Conformations = stereoisomers that can rapidly interconvert by rotation II. Chirality = describes objects not superimposable with their mirror image A) Products of Radical Bromination of Butane 1) 2) 3) 4) 5) 2 products are produced Are they mirror images? Are they Superimposable? Chiral, Enantiomeric products; each enantiomer is one of a pair (Achiral = is superimposable with mirror image) One carbon is bonded to 4 different: Me, Et, H, Br a) These carbons are always chiral b) Asymmetric carbon A A c) Stereocenter D C B B D C B) Molecular Symmetry 1) Plane of Symmetry a) b) c) d) 2) Bisects molecule to give mirror image halves Chiral molecules have zero planes of symmetry Methane has 6 planes of symmetry: Achiral CHFClBr has no planes of symmetry: Chiral H H H When is Chiral not Chiral? a) Rapid structural changes give an Average Symmetry b) A given structure must be room temperature isolatable c) Conformational changes i. Butane conformations are chiral, but Butane is achiral ii. cis-1,2-dimethylcyclohexane has achiral intermediate H III. Optical Activity A) How do you tell Enantiomers Apart? 1) Most Physical Properties are identical: mp, bp, density, etc… 2) Enantiomers interact differently with plane polarized light 3) Each enantiomer of a pair rotates the light in a different direction a) Dextrorotatory = clockwise rotation (+) b) Levorotatory = counterclockwise rotation (-) 4) Descriptions: Optical Activity, Optically Active, Optical Isomers B) a Using a Polarimeter to calculate Optical Purity to 1) a, the rotation angle, depends on cell length, T, solvent, etc… a) L = length in dm (0.1 m) c = concentration in g/ml b) Specific rotation can be compared; constant for given molecule 2) 3) 4) 5) Pure enantiomer has maximum a (+23o or –23o) Racemic Mixture = 50%/50% mixture of enantiomers has a = 0 Anywhere in between will have rotation between these values Enantiomeric Excess = %Optical Purity = how much more of one enantiomer than the other a %Optical Purity observed x100 a 6) Exercise 5-7 75% optical purity, [a] = 23.1o, what is [a]observed? 0.75 7) ao 0.75a a o (0.75)( 23.1o ) 17.33o a How much of each enantiomer is present? NOT 75%/25% 12.5% (+) / 12.5% (-) and 75% (+) Total = 87.5% (+), 12.5% (-) a lc IV. R,S Rules A) Absolute Configuration 1) +/- tell us the interaction of light, not the exact structure of enantiomers A D C 2) B) A B B D C X-Ray Crystallography gives us Absolute Configuration a) Crystals are regularly arranged solid forms b) They Diffract X-rays regularly, so we can tell what atom is where c) X-Ray and Polarimetry lets us match +/- with a specific structure d) Similar molecules usually have same +/- correlation R/S Labels 1) Cahn-Ingold-Prelog System assigns name to each enantiomer 2) Arrange substituents with lowest priority in back a) Clockwise arrangement high-to-low = R (rectus = right) b) Counterclockwise = S (sinister = left) R enantiomer S enantiomer 3) 4) Write the name: R-(+)-chloroflouorobromomethane Racemic Mixture: R,S-(+/-)-chlorfluorobromomethane 5) Rules for assigning priority (Try Exercise 5-8 thru 5-10) a) Highest Atomic Number: Br > Cl > F > H b) For equal atomic number, look at what is attached i. Only look at one level at a time ii. If second level identical, then go to third level c) Treat double and triple bonds as 2 or 3 single bonds to that atom H a c ClCH2 CH3CH2 H a Cl,2H > C,2H > 3H CH3 b c (CH3)2CH CH3CH2 2C,H > C,2H > 3H CH3 b a HOCH2CH2 CH3CH2 c H a H CH2CH2CH3 b HC C CH3CH2 c b CH CH2