Lecture Notes
advertisement

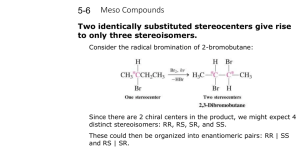
Chapter Eighteen Carbohydrates Carbohydrates cont’d Ch 18 | # 2 of 52 Carbohydrates • Major source of ______ from our diet • Composed of the elements _, _ and _ • Produced by photosynthesis in plants • Also called saccharides (sugars) Ch 18 | # 3 of 52 Carbohydrates, Chemically • Polyhydroxy aldehydes or polyhydroxy ketones O H2C OH C O CH HC HO OH CH HC HC HC OH HC CH3 OH OH HC HC OH OH OH CH3 Ch 18 | # 4 of 52 Monosacchrides • Three Carbons = ________ • Four Carbons = ________ • Five Carbons = ________ • Six Carbons = ________ Ch 18 | # 5 of 52 Monosacchrides • Aldoses are monosacchrides with an _________________ group and many hydroxyl (-OH) groups. • Ketoses are monosacchrides with a __________________ group and many hydroxyl (-OH) groups. Ch 18 | # 6 of 52 Glucose Ch 18 | # 7 of 52 Galactose D-galactose Ch 18 | # 8 of 52 Fructose Ch 18 | # 9 of 52 Types of Carbohydrates • Monosacchrides A carbohydrate that contains a single polyhydroxy aldehyde or polyhydroxy ketone unit. • Disaccharides Contain 2 monosacchride units • Polysacchrides Contain many monosacchride units Ch 18 | # 10 of 52 Different Forms of Monosaccharides • Most monosaccharides exist in two different forms – “left-handed” and – “right-handed” • The two forms are related to each other the same way that mirror images are related to each other. Ch 18 | # 11 of 52 Carbohydrates cont’d ← Fig. 18.3 The mirror image of the right hand is the left hand. Conversely, the mirror image of the left hand is the right hand. Ch 18 | # 12 of 52 Carbohydrates cont’d → Fig. 18.4 A person’s left and right hands are not superimposable upon each other. Ch 18 | # 13 of 52 Mirror Images • Mirror images – The reflection of an object in a mirror • Superimposable mirror images – Images that coincide at all points when the images are laid upon each other • Nonsuperimposable mirror images – Images where not all points coincide when the images are laid upon each other • The easiest way to determine if two mirror images are superimposable or not, is to make models Ch 18 | # 14 of 52 Chiral Objects • Chiral molecules have mirror images that are not superimposable • Chiral molecules contain at least one chiral center • A chiral carbon atom (“chiral center”) has four different groups attached to it (typically marked with *) Ch 18 | # 15 of 52 Carbohydrates cont’d Fig. 18.5 Examples of simple molecules that are chiral. Ch 18 | # 16 of 52 Stereoisomerism • Structural isomers – Isomers in which atoms are connected to each other in different ways • Stereoisomers – Isomers whose atoms are connected in the same way but which differ in the orientation of these atoms in space • Stereoisomers always have a chiral center and structural rigidity • Cis-trans isomers are one form of stereoisomerism Ch 18 | # 17 of 52 Stereoisomers • 2 different kinds of stereoisomers – Enantiomers • Stereoisomers whose molecules are nonsuperimposable mirror images of each other (the left- and right-hand versions of a single molecule) – Diastereomers • Stereoisomers whose molecules are not mirror images of each other Ch 18 | # 18 of 52 Carbohydrates cont’d → Fig. 18.6 Enantiomers are stereoisomers whose molecules are nonsuperimposable mirror images of each other. a) Enantiomers b) Diastereomers – molecules are not mirror images Ch 18 | # 19 of 52 Carbohydrates cont’d ← Fig. 18.7 The “thought process” used in classifying molecules as enantiomers or diastereomers. Ch 18 | # 20 of 52 Ch 18 | # 21 of 52 Carbohydrates cont’d → Fig. 18.8 Emil Fischer was one of the early greats in organic chemistry. He is credited with the development and use of the Fischer Projection Formulas. We have already used this notation in the tetrahedral drawing in a two dimensional form. See page 519 in your text. Edgar Fahs Smith Collection, University of Pennsylvania Library Ch 18 | # 22 of 52 Fischer Projections • A two-dimensional structural notation for showing the spatial arrangement of groups about chiral centers in molecules – Vertical lines = bonds to groups directed into the page (away from you) – Horizontal lines = bonds to groups directed out of the page (towards you) H O O CH CH C CH3 OH HO C H CH3 Ch 18 | # 23 of 52 Properties of Chiral Centers • Most important property is a solution of a stereoisomer is its ability to rotate plane polaraized light. • Light passing through polarized filters has waves only in one direction, i.e., the light is polarized. Ch 18 | # 24 of 52 Carbohydrates cont’d ← Fig. 18.9 Vibrational characteristics of ordinary and polarized light. Ch 18 | # 25 of 52 Carbohydrates cont’d Fig. 18.10 Schematic depiction of how a polarimeter works. Ch 18 | # 26 of 52 • Rotation of light: – Levorotatory: Rotation of light to the left – Dextrorotatory: Rotation of light to the right • Not to be confused with D and L forms or the “handedness” of molecules. They are different. Ch 18 | # 27 of 52 D notation Ch 18 | # 28 of 52 Chiral Centers – Why Do We Care? • Monosaccharides often contain more than one chiral center – This means that there are at least two different forms of each monosaccharide, a left-handed form and a righthanded form – Each form elicits a different chemical response • Biologically active monosaccharides are the righthanded versions of molecules Ch 18 | # 29 of 52 Carbohydrates cont’d ← Fig. 18.11 The distinctly different natural flavors of spearmint and caraway are caused by enantiomeric molecules. Ch 18 | # 30 of 52 Intermolecular Interactions • Interactions where there is binding or contact at three points. • Look at enantiomeric pair and see how each would bind differently. Ch 18 | # 31 of 52 Carbohydrates cont’d → Fig. 18.12 Epinephrine binds to the receptor at three points. Ch 18 | # 32 of 52 Ch 18 | # 33 of 52 Ch 18 | # 34 of 52 Cyclic Structures • Monosaccharides with 5-6 carbon atoms form cyclic structures • The hydroxyl group on C-5 reacts with the aldehyde group or ketone group (to form a hemiacetal or a hemiketal) Ch 18 | # 35 of 52 Haworth Structure for D-Isomers • Two-dimensional structural notations that specifies the 3-dimensional structure of a cyclic form of a monosaccharide – The cyclic structure of a D-isomer has the final CH2OH group located above the ring. Ch 18 | # 36 of 52 → Fig. 18.16 The cyclic hemiacetal forms of Dglucose result from the intramolecular reaction between the carbonyl group and the hydroxyl group on carbon 5. Ch 18 | # 37 of 52 Haworth Structure for D-Glucose -D-Glucose -D-Glucose Ch 18 | # 38 of 52 Carbohydrates cont’d ← Fig. 18.17 Walter Norman Haworth was a British carbohydrate chemist. © Hulton-Deutsch Collection / CORBIS Ch 18 | # 39 of 52 → Fig. 18.19 The three forms of maltose present in aqueous solution. Ch 18 | # 40 of 52 Disaccharides • A disaccharide consists of two monosaccharides • Glucose + Glucose Maltose + water • Glucose + Galactose Lactose + water • Glucose + Fructose Sucrose + water Ch 18 | # 41 of 52 Ch 18 | # 42 of 52 ← Fig. 18.22 The polymer chain of a polysaccaride may be unbranched or branched. Ch 18 | # 43 of 52 Amylose, Amylopectin, and Glycogen • Amylose is a continuous chain of glucose molecules linked by -1,4 glycosidic bonds • Amylopectin is a branched chain of glucose molecules linked by a -1,4- and -1,6-glycosidic bonds. • Glycogen is similar to amylopectin, but more highly branched Ch 18 | # 44 of 52 Fig. 18.24 Two perspectives on the structure of the polysaccaride amylopectin. Ch 18 | # 45 of 52 Cellulose • Cellulose is a polymer of glucose molecules linked by -1,4-glycosidic bonds • Enzymes in saliva can hydrolyze -1,4 glycosidic bonds in starch, but not -1,4 glycosidic bonds in cellulose Ch 18 | # 46 of 52 Carbohydrates cont’d Ch 18 | # 47 of 52 Carbohydrates cont’d Fig. 18.28 The structures of cellulose (a) chitin (b). Ch 18 | # 48 of 52 Phosphate Ester Formation • When a cyclic monosaccharide reacts with phosphoric acid, a phosphate ester is produced • Phosphodiesters link the monomers in DNA Ch 18 | # 49 of 52 Amino Sugar Formation • When a cyclic monosaccharide reacts with an amine, an amine sugar is produced Ch 18 | # 50 of 52 Glycolipids and Glycoproteins • A glycolipid is a lipid molecule that has one or more carbohydrate (or carbohydrate derivative) units covalently bonded to it. • A glycoprotein is a protein molecule that has one or more carbohydrate (or carbohydrate derivative) units covalently bonded to it. Ch 18 | # 51 of 52 Ch 18 | # 52 of 52