Antidiabetic Drugs
advertisement

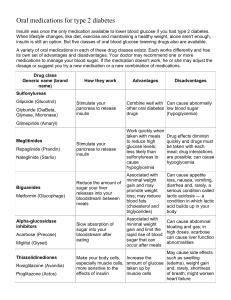
Antidiabetic Drugs Kaukab Azim, MBBS, PhD Drug List - INSULINS Preparation Onset of effect Peak Activity duration (hours) Rapid-acting insulins Insulin Lispro ☛ 10 – 20 minutes Insulin Aspart ☛ Short-acting insulins Regular insulin ☛ 30 – 60 minutes 30 – 60 minutes 3–5 1 – 2 hours 5–7 6 – 12 hours 18 – 24 16 – 18 hours No peak 24 – 36 24 Intermediate-acting NPH (Neutral ☛ 1 – 2 hours protamine Hagedorn) Insulin Lente ☛ Long-acting insulins Insulin Ultralente ☛ 4 – 6 hours Insulin glargine ☛ 1 – 2 hours Note: All are administered subcutaneously; Regular insulin can be administered I.V., especially in the management of diabetic ketoacidosis; surgery and during acute infections. Drug List – Oral Antidiabetics Insulin secretagogues Biguanides Sulfonylureas Thiazolidinediones Meglitinides α-glucosidase inhibitors Tolbutamide* Chloroprapamide* Glyburide** Glipizide** Repaglinide Metformin Rosiglitazone Acarbose Miglitol Glimepiride** (amaryl) * 1st generation sulfonylureas, ** 2nd generation sulfonylureas (GLIP ih zyd), (glye-MEP-ir-ide), (met FOR min), (thy-a-zoll-i-deen-dye-ones) Learning Outcomes ✐ ✐ ✐ ✐ ✐ ✐ ✐ ✐ ✐ ✐ ✐ ✐ ✐ ✐ ✐ ✐ ✐ List the main factors regulating insulin secretion. Explain the mechanism of action of insulin and glucagon. Explain the mechanism of action of each class of oral antidiabetic drugs. Describe the major effects of insulin upon carbohydrate, lipid and protein metabolism. Contrast the actions of insulin and the counter regulatory hormones on liver and muscle. Describe the major effects of oral antidiabetic agents. List the main factors affecting insulin absorption. List the routes of administration and the duration of action of various insulin preparations. List the duration of action of the oral antidiabetic agents. Describe the adverse effects of insulin, glucagon,. and oral antidiabetic agents Describe the clinically relevant interactions between antidiabetic drugs and other drugs. List the main contraindications of antidiabetic drugs. Explain the choice of an insulin regimen in diabetics. List the main events requiring an increase in the dosage of insulin in the diabetic patient. Outline the use of oral antidiabetic agents in the treatment of diabetes mellitus. List other therapeutic uses of sulfonylureas. List the therapeutic uses of glucagon. Insulin Synthesis and Secretion Synthesis Golgi apparatus of B cells synthesizes insulin from proinsulin. Insulin is stored in secretory granules. Secretion a. b. Basal release (in pulses every 15-30 minutes) Glucose stimulated release: 1. Early, rapid phase (stored insulin is secreted) 2. Later, slower phase (newly synthesized insulin is secreted). A Simplified Model of Glucose stimulated release of Insulin 1 2 Metabolism of glucose increases intracellular ATP 3 ATP closes ATP-dependent K+ channels 4 Decrease efflux of K+ causes depolarization Depolarization opens voltagegated Ca++ channels 5 Ca++ triggers insulin release by exocytosis Factors Regulating Insulin Secretion Factor Increased Secretion Decreased Secretion Alimentary Certain amino acids (leucine, arginine) Fatty acids Metabolic Hyperglycemia* Hyperkalemia Hypoglycemia Hypokalemia Hormonal Intestinal hormones (glucagon-like peptide, gastrin, secretin, VIP, cholecystokinin) Somatostatin Amylin Neural Sympathetic stimulation (beta2 receptors) Vagal stimulation (muscarinic receptors) Sympathetic stimulation (alpha2 receptors) Beta2 agonists Sulfonylureas Meglitinides Alpha2 agonists Phenytoin Ca++ blockers Loop diuretics Thiazide Diazoxide Drugs** * Hyperglycemia is the only factor that stimulates both phases of insulin secretion ** Many drugs can affect secretion indirectly by causing hyper or hypoglycemia Mechanism of Action of Insulin ✐ Insulin binds to a specific transmembrane tyrosine-kinase linked receptor located in cell membranes of most tissues. The receptor consists of two alpha subunits linked to two beta subunits. (the affinity of insulin for its receptor is lowered by corticosteroids and increased by growth hormone; at concentration of insulin that produce maximal effects only 10% of the receptors are occupied) ✐ Insulin binding to the alpha subunits causes the activation of the beta receptor subunit, which contain the tyrosine kinase. The enzyme is phosphorylated and this turn leads to the following two cascade pathways: 1. 2. IRS-1 Pathway IRS-2 Pathway Mechanism of Action of Insulin The insulin receptor ✐ A specific transmembrane tyrosine-kinase linked receptor located in cell membranes of most tissues. ✐ Activation of this receptor, triggers the phosphorylation of a tyrosine kinase enzyme which in turn leads to the following two cascade pathways: 1. Insulin receptor substrate-1 (IRS-1) pathway: Leading to a. b. Regulation of proliferation and differentiation of several cell types Regulation of DNA synthesis 2. Insulin receptor substrate-2 (IRS-2) pathway: Leading to a. b. c. Increased glucose uptake by the lipid and muscle cells Increased glycogen formation Regulation of gene transcription Pharmcodynamics of Insulin The general physiological function of insulin is to conserve fuel by facilitating the uptake, utilization and storage of glucose, amino acids and fats after meals. Effects on carbohydrate metabolism ✐ Increased glucose transport into the cells (several glucose transporters are activated). ✐ Increased glycogen synthesis (glycogen synthase is stimulated) ✐ Increased glycolysis (the activity of several key enzymes is stimulated) ✐ Decreased glycogenolysis (glycogen phosphorylase is inhibited). ✐ Decreased gluconeogenesis (many gluconeogenic enzymes are depressed). Pharmcodynamics of Insulin The ultimate effect of insulin is to control the intracellular utilization of glucose, as follows: ✐ 50% of ingested glucose is converted to energy (glycolysis) ✐ 10% of ingested glucose is converted to glycogen (glycogen synthesis) ✐ 40% of ingested glucose is converted to fat Effects on lipid metabolism ✐ Increased triglyceride formation and storage (lipoprotein lipase is induced and activated to hydrolyze triglycerides from lipoproteins. Glycerol phosphate generated from glucose permits esterification of fatty acids). ✐ Decreased lipolysis (direct inhibition of hormone-sensitive intracellular lipase) ✐ Increased lipogenesis (glucose is converted to fat) Pharmcodynamics of Insulin Effects on protein metabolism ✐ Increased amino acid transport into the cells. ✐ Increased protein synthesis. Other metabolic effects ✐ Increased transport into cells of K+, Ca++, nucleosides and phosphate. Long-term actions ✐ Stimulation of cell proliferation SUMMARY OF MAIN INSULIN EFFECTS ON CARBOHYDRATE, FAT, AND PROTEIN METABOLISM IN LIVER, ADIPOSE TISSUE AND MUSCLE Liver Adipose Tissue Muscle Glucose uptake Glycogen synthesis Sugar Glycolysis Glycogenolysis Glucose uptake Glycerol synthesis Glucose uptake Glycolysis Glycogen synthesis Gluconeogenesis Fat Lipogenesis Triglyceride synthesis Lipolysis Lipolysis Amino acid uptake Protein Protein breakdown Protein synthesis Protein breakdown Increases Decreases Pharmacokinetics of Insulin ABSORPTION ✐ ✐ ✐ ✐ Bioavailability: NO ORAL BIOAVAILABILITY SC, IM: good. Nasal: good (investigational). DISTRIBUTION ✐ Bound in plasma: < 5%. ✐ Vd (70 Kg): . 15 L. BIOTRANSFORMATION ✐ All insulin is metabolized in liver, kidney, and muscle (internalized with insulin receptors and destroyed intracellularly) ✐ (50% of insulin secreted by pancreas into the portal vein does not reach the general circulation). EXCRETION ✐ None ✐ Total Clearance: 800-2500 mL/min (70 Kg) Half-life: 5-10 minutes Diabetes Mellitus Definition A syndrome characterized by hyperglycemia resulting from impaired insulin secretion and/or effectiveness, associated with risks for diabetic ketoacidosis (DKA) or nonketotic hyperglycemic-hyperosmolar coma (NKHHC), and a group of late complications including retinopathy, nephropathy, atherosclerotic coronary and peripheral arterial disease, and peripheral and autonomic neuropathies. Different Types of Diabetes General Both genetic and environment factors are involved in causation of diabetes a. Type I (formerly called insulin-dependent) b. A serious form of diabetes characterized by destruction of pancreatic beta cells and by severe or absolute insulin deficiency. Type II (formerly called non insulin-dependent) A milder form of diabetes characterized by tissue resistance to the action of insulin combined with a relative deficiency of insulin secretion. Specific c. Type III (also called secondary) d. Diabetes secondary to other diseases (Cushing’s disease, acromegaly, chronic pancreatitis, genetic syndromes, etc.) or drug therapy. Type IV (also called gestational diabetes) Any abnormality in glucose level noted for the first time during pregnancy (it occurs in about 4% of all pregnancies in USA) Characteristics of Major Types of Diabetes Mellitus Type 1 Type 2 % of all cases 10 – 20 80 – 90 Age of onset (years) Generally < 30 Generally > 30 Associated obesity No Very common Propensity for DKA Yes No Endogenous insulin secretion Extremely low Significant but variable Islet cell antibodies Yes No Islet pathology Loss on most beta cells Smaller, normal, appearing islets Associated risks Present Present Response to sulfonylureas No Yes, initially in many patients Insulin Therapy All available insulin preparations are either human insulin (produced by recombinant DNA techniques) or human analog insulin (some amino acids in the molecule are substituted or changed in position) Main insulin preparations 1. Lispro (human insulin analog): two amino acids near the end chain have been reversed in position. 2. Aspart (human insulin analog): proline is substituted with aspartic acid at the B28 position. Regular: crystalline insulin-zinc (IZ) salt solution. 3. 4. 5. 6. 7. NPH (Neutral Protamine Hagedorn): suspension of insulin in a complex with zinc and protamine. Lente: suspension of large IZ particles. Glargine: glycine is substituted for asparagine at the A21 position and two arginine molecules are attached to the B chain. Ultralente: suspension of very large IZ particles. Insulin Therapy Administration Insulin is administered either IV, IM or SC. Administration of insulin differs from physiological secretion of insulin because: a. The kinetics does not reproduce the normal rapid rise and decline of insulin secretion in response of ingestion of nutrients. b. The insulin diffuses into the peripheral circulation instead of being released into the portal circulation. Therefore the direct effects of insulin on hepatic metabolic processes are eliminated. Insulin Therapy Factors affecting SC insulin absorption The site of injection (absorption is most rapid from the abdominal wall, followed by the arm, buttock and thigh). The deep of injection (IM absorption is faster than SC absorption). The type of insulin. Subcutaneous blood flow (in the upright posture sc blood flow diminish considerably in the legs) Regional muscular activity at the site of injection. Volume and concentration of the injected insulin (a large volume can lead to an initial "lag phase" of absorption) Insulin Therapy The duration of action of insulin can be varied by: 1. Modification of the insulin molecule (by recombinant technology) 2. Conjugation of insulin with protamine in a low soluble complex. After injection proteolytic enzymes degrade protamine so allowing absorption of insulin. 3. Combination of insulin with zinc, to form zinc salts. After injection the salt precipitates and insulin is slowly released. Duration of Action of Insulin Preparations Type Administration Action Onset* Peak Length IV, SC IV, SC 15 Min 15 Min 1–2h 1h 3–4h 3–5h IV, SC 45 Min 1.5 – 4 h 5–8h SC SC 1–2h 1–2h 6 – 12 h 6 – 12 h 16 – 24 h 16 – 24 h SC SC 4–6h 1–2h 16 – 18 h 4–5h 20 – 36 h > 24 h Ultra-rapid-acting Lispro insulin Aspart insulin Rapid-acting Regular insulin Intermediate-acting NPH insulin (isophane) Lente insulin Long-acting Ultralente insulin Glargine insulin ☛ Ultra-rapid-acting insulins permit a more physiologic prandial insulin replacement. They can be taken 5 minutes before meal and their short duration of action decreases the risk of late postmeal hypoglycemia ☛ Regular insulin is the only insulin that can be administered IV. It is particularly useful (given by IV infusion) for the management of diabetic ketoacidosis * Onset refers to SC administration Adverse Reactions to Insulin Hypoglycemia ✐ It is the most common complication of insulin therapy. ✐ It can be also due (in long-term diabetics) to an inadequate production of counterregulatory hormones that normally provide an effective defense against hypoglycemia. Symptoms and signs They are first discerned at a plasma level of 60 to 80 mg/DL and include: 1. Signs of autonomic hyperactivity. Both sympathetic (tachycardia, sweating, tremulousness, anxiety) and parasympathetic (hunger, nausea) 2. Signs of impaired function of the central nervous system. They are also named neuroglycopenic symptoms (headache, mental confusion, weakness, dizziness, blurred vision, drowsiness, bizarre behavior, convulsions and coma). Therapy • • Conscious patients: oral glucose Unconscious patients: IV glucose or glucagon IM Adverse Reactions to Insulin Immunological problems Allergic reactions ✐ They are generally mediated by IgE antibodies and are often due to noninsulin protein contaminants. Immune insulin resistance ✐ It is exceedingly rare with human purified insulin. Local reactions at the injection sites ✐ Hypertrophy of subcutaneous fatty tissue can occur after month of repeated injections on the same site (it remains a problem ,even with purified insulin) ✐ Atrophy of subcutaneous fatty tissues (rare today). ✐ Localized infections. Interaction of Insulin with Other Drugs Drug Interaction Clinical Relevance Alcohol Hypoglycemia (ethanol inhibits gluconeogenesis) High Beta-blockers Prolonged hypoglycemia and masking of certain symptoms of hypoglycemia High Salicylates Hypoglycemia, with large doses (mechanism unknown) Medium Fenfluramine Hypoglycemia (the drug increases the uptake of glucose into striated muscle) Medium MAO inhibitors Hypoglycemia (MAO inhibit gluconeogenesis) Medium Glycemic Control in Diabetes ✐ The short-term benefits of tight blood glucose control in diabetics are well established. ✐ Recent evidence (DCCT Research Group, 1993) indicates that meticulous blood glucose control can also dramatically reduce and slow the development of tissue complications in type 1 diabetes. ✐ Patients receiving meticulous blood glucose control however have a threefold greater risk of severe hypoglycemic episodes. ✐ The consensus of the ADA is that tight blood glucose control should become standard therapy in type I as well as in type II diabetes after the age of puberty. ✐ BEWARE OF HYPOGLYCEMIA Events Requiring an Increase in Dosage of Insulin in Diabetic Patients ✐ Infections ✐ High fever ✐ Trauma, surgical operations ✐ Myocardial infarction ✐ Pregnancy ✐ Hyperthyroidism ✐ Diabetic ketoacidosis Oral Antidiabetic Drugs Insulin Secretagogues Sulfonylureas Meglitinides Short-acting Tolbutamide Intermediate acting Glyburide Long-acting Chloropropamide Repaglinide Euglycemic Agents Biguanides Metformin Thiazolidinediones Pioglitazone Glucose Absorption Inhibitors Alpha-glucosidase inhibitors Miglitol Pharmacology of Sulfonylureas Mechanisms of action 1. Increased pancreatic response to glucose (the main mechanism) by: Binding to a specific receptor associated with a ATP-sensitive K+ channel in beta cell membranes (the channel is normally blocked by glucose-induced increase in ATP) Blockade of K+ efflux (depolarization) Opening of voltage-gated Ca++ channels Release of insulin by exocytosis (Insulin synthesis is not affected) 2. Reduction of plasma glucagon levels after chronic treatment (mechanism is unclear but could be related to the enhanced release of both insulin and somatostatin, which inhibit A cell secretion.) Pharmacology of Sulfonylureas Pharmacological effects ✐ Hypoglycemic effect (only if insulin is available) ✐ Stimulation of somatostatin release from pancreatic D cells. ✐ The hypoglycemic effect of sulfonylureas decreases over time (secondary failure). Pharmacokinetics of Sulfonylureas The major differences between various sulfonylureas reside in their pharmacokinetic profiles Drug Half Life Duration of Action First generation Tolbutamide 5 – 7 hours 6 – 12 hours Chloropropamide 25 – 35 hours 40 – 60 hours 3 – 4 hours 10 – 24 hours Second generation Glyburide Adverse Effects of Sulfonylureas (overall incidence of adverse effects ~ 4%) Metabolic effects • Hypoglycemic reactions (up to 20%)(more likely with compounds having longer half-lives) Allergic skin reactions • Itching (3%), skin rashes (1%), urticaria (1%). Other effects • Disulfiram-like reaction in patients ingesting alcohol (10-15%) (chlorpropamide) • Dilutional hyponatremia (1-5%), SIADH (with symptoms of water intoxication) (mainly with chlorpropamide) Drug Interactions with Sulphonylureas Hypoglycemic action is increased by: Insulin* Alcohol* Sulfonamides Probenecid Chloramphenicol Hypoglycemic action is decreased by: CorticosteroidsΩ Hormonal contraceptivesΩ Loop and thiazide diureticsΩ Rifampin✜ * Intricsic hypoglycemic action Inhibition of hepatic metabolism of sulfonylureas Inhibition of urinary secretion of sulfonylureas Ω Intrinsic hyperglycemic action ✜ Stimulation of hepatic metabolism of sulfonylureas Contraindications and Precautions of Sulfonylureas ✐ Type I diabetes (as sole therapy) ✐ Pregnancy (risk of hypoglycemia in the newborn). ✐ Severe liver or kidney disease. ✐ Sulfa drug hypersensitivity. Therapeutic Uses of Sulfonylureas THERAPEUTIC USES of SULFONYLUREAS 1. Treatment of diabetes mellitus ✏ Sulfonylureas are used to control hyperglycemia in type II diabetic patients who cannot achieve appropriate control with changes in diet alone. 2. Treatment of diabetes insipidus ✏ Chlorpropamide can reduce or eliminate the need for vasopressin in some patients with central diabetes insipidus when partial ADH secretion is present. Pharmacology of Meglitinides and congeners Drugs ✐ Repaglinide and nateglinide are the drugs on the market. Mechanism of action ✐ Stimulation of insulin release by closing ATP-dependent K+ channels in pancreatic beta cells (the mechanism is very close to that of sulfonylureas) Pharmacokinetics ✐ Repaglinide has a fast onset (less than 30 minutes) and a short duration of action (about 4 hours). Repaglinide is > 95% metabolized by the liver Pharmacology of Meglitinides and congeners Adverse effects ✐ ✐ Hypoglycemic reaction (up to 15%) Upper respiratory tract infections (10%) Contraindications and precautions ✐ ✐ ✐ Type I diabetes (as sole therapy) Severe hepatic disease. Pregnancy (risk of hypoglycemia in the newborn). Therapeutic uses ✐ To control hyperglycemia in type II diabetic patients who cannot achieve appropriate control with changes in diet alone. (unlike sulfonylureas they have a rapid onset and a short duration of action so that they are given with meals to enhance postprandial glucose utilization) Pharmacology of Biguanides Drugs ✐ Metformin is the only drug on the market in USA. Mechanism of action ✐ It is still uncertain. Proposed mechanisms include: 1. Inhibition of hepatic gluconeogenesis (likely the main mechanism) 2. Direct stimulation of glucose uptake and utilization (glycolysis) in peripheral tissues. 3. Reduction of plasma glucagon levels. Pharmacological effects ✐ Biguanides are antihyperglycemic, not hypoglycemic. They do not cause hypoglycemia, even in large doses, but they prevent postprandial hyperglycemia. Pharmacology of Biguanides Pharmacokinetics ✐ Oral bioavailability: . 60% ✐ All the drug is excreted unchanged in the urine ✐ Half-life: . 6 hours Adverse effects ✐ Anorexia, nausea and vomiting, metallic taste, abdominal discomfort, diarrhea (up to 20%) ✐ Lactic acidosis (rare but fatal in 50% of cases) (by inhibiting gluconeogenesis the drug impairs the hepatic uptake of lactic acid) ✐ Vit B12 deficiency Pharmacology of Biguanides Contraindications and cautions ✐ Type I diabetes ✐ All conditions that predispose to acidosis (alcoholism, hepatic diseases, hypoxemia, chronic hypoxic lung diseases, low calorie diet, myocardial infarction, septicemia, dehydration, major surgery, therapy with ACE inhibitors, etc.) ✐ Renal impairment (kidney function must be controlled since the drug is excreted unchanged in the urine). Therapeutic uses ✐ In type II diabetes (alone or in combination with sulfonylureas when diabetes does not respond to diet or sulfonylurea therapy alone). Pharmacology of Thiazolidinediones Drugs ✐ Pioglitazone and rosiglitazone are the drugs on the market. Mechanism of action ✐ These drugs are ‘insulin sensitizers’. ✐ They bind to a nuclear receptor (peroxisome proliferator activated receptor, PPAR), located mainly in adipose tissue, skeletal muscle and liver, which regulates the transcription of several insulin responsive genes. ✐ The overall effect is an enhancement of tissue sensitivity to insulin (that is a reduction in insulin resistance). Therefore the need of exogenous insulin is reduced. Pharmacology of Thiazolidinediones Pharmacological effects ✐ Reduction of hyperglycemia, hyperinsulinemia and hypertriglyceridemia that are characteristic of insulin-resistant states. ✐ The drugs are antihyperglycemic, not hypoglycemic. They do not cause hypoglycemia when given alone, but can prevent postprandial hyperglycemia. ✐ Clinical effect is not observed for 6 to 12 weeks. Adverse effects ✐ Diarrhea (13%) ✐ Upper respiratory tract infections (10%) ✐ Anemia (7%), Pharmacology of Thiazolidinediones Contraindications and cautions ✐ Type I diabetes ✐ Severe heart failure (because of drug-induced edema) ✐ Liver disease (the first drug of this class, troglitazone, was withdrawn from the marked because of serious liver toxicity) Therapeutic uses ✐ In combination with insulin, biguanides or sulfonylureas, in type II diabetes which exhibits insulin resistance. Pharmacology of Alpha-Glucosidase Inhibitors Drugs ✐ Miglitol and acarbose are the compounds on the market. Mechanism of action ✐ The drugs are competitive inhibitors of the intestinal brush border enzyme alpha-glucosidase involved in the breakdown of starches into simple sugars. ✐ Absorption of monosaccharides from duodenum and upper jejunum is reduced. Pharmacology of Alpha-Glucosidase Inhibitors Pharmacological effects ✐ Postprandial glucose levels is reduced both in normal and diabetic subjects, so creating an insulin sparing effect. ✐ The efficacy of the drugs is small. ✐ Hypoglycemia does not occur even in overdosage. Pharmacokinetics ✐ Oral bioavailability: acarbose 2%; miglitol >90% ✐ Elimination: miglitol > 90% by the kidney Adverse effects ✐ Flatulence (up to 40%) due to the appearance of undigested carbohydrates in the colon where they ferment, so releasing gas. ✐ Diarrhea (up to 20%), abdominal pain (7%). Pharmacology of Alpha-Glucosidase Inhibitors Contraindications and cautions ✐ Inflammatory bowel disease ✐ Gastrointestinal conditions worsened by gas or distension ✐ Renal disease Therapeutic uses ✐ Type II diabetes as monotherapy or in combination with sulfonylureas or insulin. ✐ As monotherapy in elderly patients or in patients with predominantly postprandial hyperglycemia. ☛ Note: if hypoglycemia occurs when administered with insulin or sulfonylureas, oral administration of sugars other than glucose is ineffective. Pharmacology of Glucagon Chemistry ✐ A single chain polypeptide of 29 amino acids. Mechanism of action ✐ Most glucagon effects result from activation of specific receptors which leads to an increase in adenylyl-cyclase activity and production of cAMP. Metabolic effects ✐ ✐ ✐ ✐ Stimulation of glycogenolysis. Stimulation of gluconeogenesis. Inhibition of glycogen synthesis. Inhibition of glucose oxidation. ☛ These effect are mainly on the liver. Glucagon is the most potent hyperglycemic drug. Pharmacology of Glucagon Other effects ✐ Inotropic and chronotropic effect on the heart, due to the increase in cAMP. ✐ Profound relaxation of intestinal smooth muscle (mechanism still uncertain). Pharmacokinetics ✐ Rapidly inactivated in liver, kidney and other tissues. Half-life: 3-6 min. Adverse effects ✐ Nausea and vomiting (risk of aspiration in unconscious patients) ✐ Hypotension (after IV administration) Pharmacology of Glucagon Therapeutic uses ✐ For the emergency treatment of severe hypoglycemic reactions (but high doses stimulate insulin release) ✐ For reversing the cardiac effect of an overdose of beta-blocking agents