Chirality Center
advertisement

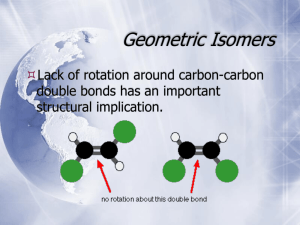
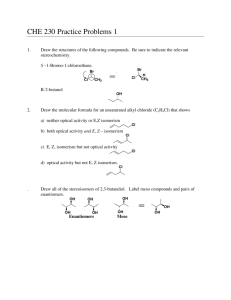
Chirality Chirality A molecule is chiral if its two mirror-image forms are not superimposable in three dimensions. The word chiral is derived from the Greek word cheir, meaning “hand”. Chirality most often occurs in molecules that contain a carbon that is attached to four different groups. Enantiomers Stereoisomers that are related as an object and its nonsuperimposable mirror image are classified as enantiomers. Rotating Molecules in Space To check whether the representations are superposable first rotate the molecule around so that the H is in the same direction. Rotating molecules in Space After rotation of B check if it is superposable with A. The H and the F are superposable but the Br and Cl are not. So, non-superposable mirror images that are enantiomers. Chirality Center Molecules of the general type CWXYZ are chiral when w, x, y, and z are different. 2-Butanol is chiral: four different groups on C-2. 2-Propanol is achiral: two of the groups on C-2 are the same. Common Chiral Molecules Linalool, a pleasant smelling oil from oranges. Limonene, a constituent of lemon oil. C-4 is the chirality center. Chirality with Isotopes Achiral alkanes can be transformed into chiral molecules by replacing H with deuterium (D) or tritium (T) as shown in this molecule: Isotopic labelling can be used to learn information about the mechanism of reactions. For example: Symmetry in Achiral Compounds A molecule that has either a plane of symmetry or, a center of symmetry will be superposable on its mirror image and therefore achiral. Plane of Symmetry A plane of symmetry bisects a molecule so that one half of the molecule is the mirror image of the other half. This achiral molecule, chlorodifluoromethane, has the plane of symmetry shown. Center of Symmetry A point is a center of symmetry if any line drawn from it to some element of the structure will, when extended an equal distance in the opposite direction, encounter an identical element. Optical Activity A sample is “optically active” if it rotates the plane of polarized light. To be optically active, the sample must contain a chiral substance and one enantiomer must be present in excess of the other. Optical Activity The direction and magnitude of rotation are cited as α, the observed rotation. Mixtures of enantiomers are characterized by the % enantiomeric excess (ee). ee = (% major enantiomer) – (% minor enantiomer) If ee = 100% the sample is enantiopure. A 1:1 mixtures of enantiomers are called racemic mixtures. Racemic mixtures are optically inactive. All achiral substances are optically inactive. Specific Rotation Specific rotation [a] is the rotation adjusted for concentration and the length of the sample cell. [a] can be used to determine the sample purity. Enantiomers have [a] of equal magnitude but opposite sign. Absolute Configuration The exact three-dimensional spatial arrangement of substituents at a chirality center is its absolute configuration. Cannot decide which is (+)-2-butanol, or (-)-2-butanol! Relative Configuration Compounds have the same relative configuration if the configuration of the chirality center is the same. In these reactions the reactant and product have the same relative configuration because the reactions do not affect the chirality center. From Relative Configuration to Absolute Configuration Absolute configuration of a salt of (+) tartaric acid was determined in 1951 by X-ray crystallography. The absolute configurations of all compounds related to (+)tartaric acid were then known. In this way the absolute configuration of the enantiomers of 2-butanol are known. The Cahn-Ingold-Prelog Rules 1. Rank the substituents at the chirality center according to rules used in E-Z notation. highest = -OH > CH2CH3 > CH3 > H = lowest 2. Orient the molecule so that lowest-ranked substituent points away from you and ignore the lowest-ranked substituent. becomes The Cahn-Ingold-Prelog Rules 3. If the order of decreasing precedence traces a clockwise path, the absolute configuration is R. If the path is counterclockwise, the configuration is S. becomes So the name is (S)-2-Butanol. Names of Enantiomers The pair of enantiomers differ only in the arrangement of atoms in space so the name only differs in the R/S assignment. Chirality Center in a Ring Remember a C=C double bond is treated like two bonds to a C. (+)-4-methylcyclohexene and the configuration is R Fischer Projections For a Fischer projection the molecule is oriented so that the vertical bonds at the chirality center are directed away from you and the horizontal bonds point toward you. The chirality center is at the center of a cross and not specified. Fischer Projections The molecule is oriented so that the lowest numbered carbon is at the top of the chain. Properties of Enantiomers Some enantiomers have different odors. Each enantiomer reacts with the receptors in the nose differently. This is chiral recognition which is common in nature where receptors interact with only one enantiomer. Chiral Drugs Many drugs have a chirality center, two examples are ibuprofen and thalidomide. Often only one enantiomer is active. (S)-Ibuprofen has pain-relieving properties while the (R)enantiomer does not. (R)-Thalidomide was used for its anti-nausea properties. The (S)-enantiomer caused birth defects. Chirality Axis A chirality axis is an axis about which a set of atoms or groups is arranged so that the spatial arrangement is not superposable on its mirror image. Substituted biaryls like biphenyl may have chirality axes and exist as two non-superposable enantiomers. Chirality Axis Unsubstituted biphenyl (A, B, X, Y = H) is nonplanar but can rotate about the C-C single bond to interchange between conformations rapidly. With substituents A, B, X, Y the conformations are locked and cannot interchange. Chirality Axis The compound below was demonstrated to exist as two enantiomers with a chirality axis in 1922! These isomers are known as atropisomers, from the Greek “a” meaning not and “tropos” meaning turn. Chirality Axis Many compounds with chiral axes are incorporated into metal catalysts. These catalysts mediate reactions that are enantiospecific and yield predominantly one enantiomer as product. Binap is one example used in chiral drug synthesis. Reactions that Create Chiral Centers Many of the reactions we have examined so far often form chiral centers. The central carbon of the epoxide is a chirality center with four different groups attached. Reactions that Create Chiral Centers The alkene is planar so the peroxyacid attacks the alkene from the top or the bottom. Reactions that Create Chiral Centers Addition of HBr to an alkene can generate a chirality center. Chlorination of alkanes can generate a chirality center. Both reactions form a 1:1 mixture of enantiomers which is a racemic mixture. Reactions that Create Chiral Centers Optically inactive starting materials can give optically active products only if they are treated with an optically active reagent or if the reaction is catalyzed by an optically active substance. In nature the chiral catalyst is an enzyme. Molecules with two Chirality Centers Consider 2,3-dihydroxybutanoic acid. Carbons 2 and 3 are chirality centers. Each stereocenter could be R or S so there are four possible stereoisomers: (2R, 3R); (2S, 3S); (2R, 3S); (2S, 3R). Stereoisomers of 1,2-Dihydroxybutanoic acid Stereoisomers I and II are enantiomers of each other; Stereoisomers III and IV are enantiomers of each other; Stereoisomers of 1,2-Dihydroxybutanoic acid Stereoisomers I and III are diastereomers of each other. Diastereomers are stereoisomers that are not enantiomers. Pairs of diastereomers: I and III; I and IV; II and III; II and IV. Stereoisomers of 1,2-Dihydroxybutanoic acid Enantiomers: Are mirror images of each other. With two stereocenters in a molecule the enantiomer has the configuration changed at both stereocenters. Enantiomers have equal and opposite specific rotations. Diastereomers: Are stereoisomers that are not mirror images. With two stereocenters in a molecule a diastereomer has the configuration changed at only one stereocenter. Diastereomers will have different magnitude and direction specific rotations. Fischer Projections of 1,2-Dihydroxybutanoic Acid Setting up the Fischer Projection: Draw the structure so that the lowest numbered carbon is at the top. Arrange horizontal bonds to be facing towards you. Then flatten the molecule and draw it out. Fischer Projections of 1,2-Dihydroxybutanoic Acid Fischer Projection of compounds with multiple stereocenters simplifies identification of enantiomers and diastereomers. Erythreo isomers have like substituents on the same side. Threo isomers have like substituents on opposite sides. Physical Properties of Diastereomers Physical properties of enantiomers are identical except for the rotation of plane polarized light. Diastereomers may differ in any physical property, for example: Stereoisomers of 1-bromo-2-chlorocyclopropane Two stereocenters in a ring may also give rise to four stereoisomers which can be grouped as two pairs of enantiomers. Symmetric Molecules with two Chirality Centers 2,3-Butanediol has two chirality centers that are equivalently substituted. There are only three stereoisomers not four. Why? Stereoisomers of 2,3-Butanediol These are the three stereoisomers. Compound (c) has a plane of symmetry and is superposable on its mirror image. This is a molecule with chirality centers that is achiral (not chiral) and is named a meso form. It is superposable on its mirror image so there are only three stereoisomers. Stereoisomers of 2,3-Butanediol These stereoisomers can be shown as Fischer projections. The dashed line represents the plane of symmetry. Stereoisomers of 1,2-dibromocyclopropane This molecule has three stereoisomers. The cis compound has a plane of symmetry and is a meso compound. Molecules with Multiple Chirality Centers A molecule with n stereocenters can have a maximum of 2n stereoisomers. This carbohydrate has 4 stereocenters and no planes of symmetry so there are 24 = 16 stereoisomers. Molecules with Multiple Chirality Centers Steroids also contain multiple stereocenters. Cholic acid shown here has 11 stereocenters and potentionally 211 or 2048 stereoisomers. Only one has been isolated from natural sources. Alkenes and Chirality Centers Molecules that include both an alkene and a chirality center may exist as 4 stereoisomers (R,E), (R,Z), (S,E) and (S,Z). For example 3-penten-2-ol: -enantiomers- -enantiomers- Reactions that Produce Diastereomers Reaction of 2-butene with bromine yields 2,3-dibromobutane which has two identically substituted chirality centers. The product can therefore exist as a pair of enantiomers and a meso compound. The actual product formed depends on the stereochemistry of the alkene and the anti addition of bromine. Effect of Alkene Stereochemistry Anti addition of bromine to the (E) stereoisomer yields the meso product. meso-2,3-dibromobutane meso-2,3-dibromobutane A single achiral product is formed! Effect of Alkene Stereochemistry Anti addition of bromine to the (Z) stereoisomer yields a (1:1) racemic mixture of enatiomers. (2R,3R)-2,3-dibromobutane (2S,3S)-2,3-dibromobutane A 1:1 (racemic) mixture of enantiomers is formed. Generating a Second Chirality Center Hydrogenation of the alkene below yields two stereoisomers. The major product corresponds to hydrogenation from the least hindered side of the alkene. The reaction is stereoselective – one stereoisomer is formed as the major product. Resolution with Tartaric Acid The first resolution was carried out by Louis Pasteur in 1848 using tartaric acid. Pasteur separated a salt of a rare racemic mixture of tartaric acid based using a micrsocope and tweezers. Resolution of Enantiomers Enantiomers only differ in the rotation of plane polarized light but diastereomers may differ in other physical properties and may be separated. The strategy: (1) Transform the mix of enantiomers into a mix of diastereomers as a salt for separation. (2) Separate the diastereomeric mixture; (3) Reform the separated enantiomer. Resolution of Enantiomers Graphical representation. Forming Diasteromeric Salts Enantiomers are transformed into diastereomeric salts by acid-base reactions. The compounds used for resolution are generally derived from natural sources, for example (S)-malic acid from apples. Dissociating the Diastereomeric Salts After separation of the diastereomeric salts the separated salts are treated with base to regenerate the enantiomer: Kinetic Resolution Kinetic resolution depends on the different rates of reactions of two enantiomers with a chiral compound (enzyme). One enantiomer of the ester is hydrolyzed preferentially. The product of that reaction is isolated as a pure enantiomer while the unreacted enantiomer can be isolated as well.