NAME: DATE: EMPIRICAL FORMULA of MAGNESIUM OXIDE
advertisement

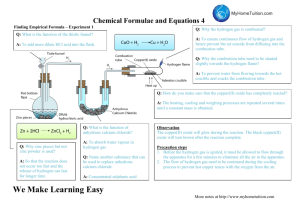
NAME: DATE: EMPIRICAL FORMULA of MAGNESIUM OXIDE INTRODUCTION In this experiment, you will determine the empirical formula of magnesium oxide, a compound that is formed when magnesium metal reacts with oxygen gas. In determining this empirical formula, you will make use of the law of conservation of mass. According to this law, the total mass of the products of a chemical reaction must equal the total mass of the reactants. mass of Mg + mass of O2 = mass of MgxOy Therefore, knowing the mass of magnesium used and the mass of magnesium oxide produced in this reaction, you can determine the mass of oxygen used. This ratio between the number of moles of magnesium used and the number of moles of oxygen consumed can then be calculated and the empirical formula of magnesium oxide can be written on the basis of this ratio. Whether it is alone or in air, molecular oxygen, O2, is a very reactive substance when it is heated and many elements will react with it. When an element reacts with molecular oxygen, it chemically combines with the oxygen and forms an oxide. Since you are going to burn the magnesium in the presence of air, you will not only form magnesium oxide, but you will also be forming magnesium nitride. Since magnesium is an action metal, it will react with both molecular oxygen and nitrogen in the air. The amount of magnesium oxide produced will be much greater than the amount of magnesium nitride. However, you will still have to convert that small amount of magnesium nitride to magnesium oxide. You will do this by adding water and then heating. The water will convert the magnesium nitride to magnesium oxide and the nitrogen will be liberated as ammonia. Heating will convert magnesium hydroxide to magnesium oxide by losing water vapor. Now your product will consist solely of magnesium oxide. PRELAB 1. Why will the crucible and contents gain mass after being heated in the presence of air? 2. Why is it hazardous to look directly at the crucible and contents during the first heating? 3. Why is it necessary to add water to the products after the first heating? 4. Based on the most common charges of magnesium and oxygen, what is the predicted empirical formula for the magnesium oxide product? What is the formula for magnesium nitride (formed as an unwanted byproduct)? 5. List the calculations that will be necessary to find the empirical formula of the product under your data table in the lab. MATERIALS Magnesium ribbon Crucible Crucible tongs Clay triangle Ring and ring stand Water in a dropper pipet Bunsen burner Scissors Electronic balance NAME: DATE: PROCEDURE 1. 2. 3. 4. 5. 6. 7. 8. 9. Obtain a crucible and lid. Clean by heating with the Bunsen burner for 2 minutes. Cool completely and find the mass of the crucible and lid. Obtain a 5-cm length of magnesium ribbon and clean the surface with steel wool (or a rough paper towel). Loosely coil the magnesium ribbon and place it in the bottom of the crucible. Determine the combined mass of the crucible, lid, and the magnesium. Record this mass in the data table. CAUTION: Do not look directly at the burning magnesium. The intense light may hurt your eyes. Place the crucible, without its lid, on the clay triangle. Heat the crucible strongly until the magnesium ignites. CAUTION: Be careful to keep the crucible at arm’s length at all times. Do not inhale the “smoke” produced. When the magnesium begins to burn, immediately place the cover on the crucible (using tongs) and remove the burner. After the reaction has subsided and “smoke” production has ceased, replace the burner and continue to heat the crucible. Every 2 or 3 minutes, remove the burner and check the progress of the reaction by using tongs to lift the lid of the crucible. Then replace the lid and again apply heat. After 10 minutes of heating, remove the burner and check the product. When the reaction is completed, the magnesium should be completely converted to a light gray powder, magnesium oxide. Allow the crucible to cool to room temperature and then add 7 or 8 drops of distilled water using a dropper pipet. Reheat the crucible and avoid inhaling the vapor. The heating must be done gently, at first, or else the crucible will crack and you will have to start from the beginning. Finish by heating strongly for 5 minutes. Some hot magnesium will react with nitrogen in air to form magnesium nitride. Most of the magnesium reacts with oxygen to form magnesium oxide. These magnesium compounds will react with water to form ammonia gas and magnesium hydroxide. The magnesium hydroxide decomposes upon heating to magnesium oxide and water vapor. Eventually all the original magnesium ends up as magnesium oxide. If no ribbon-like material remains in the crucible, allow the crucible to cool completely If ribbon-like material remains, heat the covered crucible an additional 10 minutes, then allow the crucible to cool. Reheat the crucible for 5 minutes, cool and reweigh. If the second mass is not the same as the first, reheat until a constant mass is obtained. When the mass is constant, record it. NAME: DATE: DATA TABLE Mass of crucible and lid 25.29 g Mass of the crucible, crucible lid, and the magnesium 25.33 g Mass of the crucible, crucible lid, and magnesium oxide 25.36 g CALCULATION Show all the calculations necessary to find the empirical formula of the magnesium oxide. Include a title, work, units and sig figs for each calculation. NAME: DATE: POST LAB QUESTIONS 1. Calculate the theoretical % of magnesium in magnesium oxide. Calculate the % magnesium in your product. Based on your calculations, did you successfully make magnesium oxide (show a % error calculation to support your claim). 2. A student performing this lab made an initial observation that the surface of their magnesium ribbon was dull and white. What do you suspect was on the surface of the magnesium? 3. A student performed the lab using ½ the amount of magnesium ribbon that you did. Would they obtain the same empirical formula that you did? Explain why or why not. 4. A student determined the empirical formula of potassium oxide using the following data, show the calculations necessary to find the empirical formula. Mass of crucible and lid Mass of crucible, lid, and K Mass of crucible, lid and potassium oxide product 28.29 g 28.71 g 28.79 g 5. When the empirical formula of an ionic compound is found, it is usually the actual formula of the compound. What is the actual formula of magnesium oxide? Molecular compounds will often differ from their empirical formulas. What is the empirical formula of hydrogen peroxide, H2O2? 6. Write the complete balance reaction that occurs when magnesium combines with oxygen (diatomic) to form magnesium oxide.