Step 2 - MRS. STOTTS CHEMISTRY and NATURAL SCIENCE
advertisement

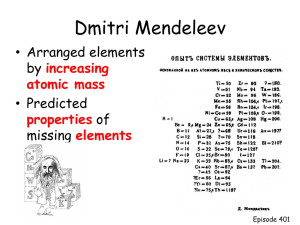
LECTURE 4 Section 1-THE PERIODIC TABLE History of Periodic Table John Newlands Placed elements in order according to their properties and in order of increasing atomic mass. Discovered the Law of Octaves-Properties appeared to repeat ever 8 elements History of Periodic Table Dmitri Mendeleev Created the first periodic table using Newlands’ information. Used all 63 known elements. Wrote the symbol, physical and chemical properties, and relative atomic mass, all on a card. Arranged until he found a pattern. Left gaps where elements had not been discovered yet and predicted their properties. History of Periodic Table Henry Moseley 40 years after Mendeleev arranged the elements, Moseley used x-rays to look at 38 different elements and their spectra, and realized that the elements were arranged by atomic number, not atomic mass. This fixed the discrepancies that were in Mendeleev’s table. The Periodic Table Periodic Law When the elements are arrange according to their atomic numbers, elements with similar properties appear at regular intervals. Valence electrons An electron that is found in the outermost shell of an atom and that determines the atom’s chemical properties. The Periodic Table Organized by the number of protons (it’s atomic number) Contains: Atomic number Symbol Name Atomic mass The Periodic Table Groups Vertical columns (↕) containing elements with similar properties Periods Horizontal rows (↔) with predictable trends Section 2- A tour-Metals On the left of the zig-zag line Make up most of the P.T. Have Luster (shiny) Good conductors of heat and electricity Malleable (can be shaped) Ductile (can be made into a wire) High tensile strength (wires can hold mass) Chapter 4 Section 2 Tour of the Periodic Table Most Elements Are Metals, continued The regions highlighted in blue indicate the elements that are metals. Chapter 4 Visual Concepts Properties of Metals: Malleability and Ductility Nonmetals Found to the right of the zig-zag line INCLUDES HYDROGEN Many are gases at room temperature Solids are brittle Poor conductors of heat & electricity Metalloids Semi-Metals Found straddling the zig-zag line Some properties of metals & nonmetals Semiconductors of electricity Chapter 4 Visual Concepts Comparing Metals, Metalloids, and Nonmetals Group Names Group 1: Alkali Metals Group 2: Alkaline Earth Metals Groups 3-12: Transition Metals Group 17: Halogens Group 18: Noble Gases Elements # 58 – 71 Lanthanides Elements # 90 – 103 Actinides Let’s Review Group 1 Alkali Metals Ends in s1 Soft, silvery metals Highly reactive Low density & melting point Let’s Review Group 2 Alkaline Earth Metals Valence shell is s2 Denser, harder, stronger than Alkali Metals Less reactive than alkali metals Let’s Review Groups 3 – 12 Transition metals Elements are a little less predictable Let’s Review Metalloids Along the staircase starting in Group 13 Valence levels p orbital Share properties of metals & nonmetals Referred to as semimetals Used as semiconductors in electronics Let’s Review Group 17 Halogens Valence level is s2 p5 Highly reactive NONmetals Forms salts when combined with Group 1 or 2 metals Let’s Review Group 18 Noble Gases Valence level is s2 p6 Usually unreactive And finally …. Lanthanides (f-block) Top Row of f-block Shiny metals similar to Group 2 Actinides Bottom Row of f-block Naturally radioactive elements TRENDS IN THE PERIODIC TABLE Section 3 What are the trends? Atomic Radius Down a group •Increases •Adding on extra electron orbitals •Stacking orbitals keep getting bigger Across a period •Decreases •More protons become ATTRACTED to more electrons Chapter 4 Section 3 Trends in the Periodic Table Atomic Radius, continued Atom’s ability to steal an e Decreases down a group Increases across a period Decreases Ionization Energy Increases Chapter 4 Section 3 Trends in the Periodic Table Ionization Energy, continued Atom’s ability to attract an e Decreases down a group Increases across a period Decreases Electronegativity Increases Atom’s ability to accept an e Decreases down a group Increases across a period Decreases Electron Affinity Increases NUCLEAR REACTIONS Chapter 4 Visual Concepts Nuclear Reaction Chapter 4 Visual Concepts Nuclear Fusion Chapter 4 Section 4 Where Did the Elements Come From? Natural Elements, continued Other Elements Form by Nuclear Reactions in Stars ELECTRON CONFIGURATIONS REFER BACK TO CHAPTER 3 SECTION 3 ELECTRONS Found in specific regions – orbital 4 orbitals s (sphere shaped) p (dumbbell shape) d (cross dumbbells) f (don’t ask… it’s complicated) s Orbitals Sphere shaped Can only hold 2 e- total p Orbitals Dumbbell shape 3 positions Each position holds 2 e6 e- total d Orbitals Cross dumbbell shape or 4 leaf clover shape 5 positions total Each position holds 2 e Holds total 10 e- f Orbitals Dumbbell shape surrounded by donut shape 7 positions Each position holds 2 e Holds total of 14 e- Using the Periodic Table Using the P.T. to Count #’s s Block Using the P.T. to Count #’s p Block Using the P.T. to Count #’s d block Using the P.T. to Count #’s f Block Chapter 4 Section 1 How Are Elements Organized? Blocks of the Periodic Table Writing Electron Configurations Orbitals must fill in a particular order The 1st number indicates the period or level the electron is found in. The letter indicates the orbital the electron is found in. The exponent indicates how many electrons are there. Sample Problem A Write the electron configuration for Sodium, Na. Sample Problem A Write the electron configuration for Sodium, Na. Step 1: Find the # of electrons (Look at the Atomic number) Na is Atomic # 11. Na has 11 e- Sample Problem A Write the electron configuration for Sodium, Na. Step 2: Follow the correct ordering and fill orbitals 11 – 2 = 9 e- left 1s2 Sample Problem A Write the electron configuration for Sodium, Na. Step 2: Follow the correct ordering and fill orbitals (9 – 2 = 7 eleft) 1s2 2s2 Sample Problem A Write the electron configuration for Sodium, Na. Step 2: Follow the correct ordering and fill orbitals (7 – 6 = 1 eleft) 1s2 2s2 2p6 Sample Problem A Write the electron configuration for Sodium, Na. Step 2: Follow the correct ordering and fill orbitals (1 – 1 = 0 eleft) DONE! 1s2 2s2 2p6 3s1 Sample Problem B Write the electron configuration for Argon, Ar. Sample Problem B Write the electron configuration for Argon, Ar. Step 1: Find the # of electrons (Look at the Atomic number) Ar is Atomic # 18. Ar has 18 e- Sample Problem B Write the electron configuration for Argon, Ar. Step 2: Follow the correct ordering and fill orbitals 18 – 2 = 16 e- left 1s2 Sample Problem B Write the electron configuration for Argon, Ar. Step 2: Follow the correct ordering and fill orbitals (16 – 2 = 14 eleft) 1s2 2s2 Sample Problem B Write the electron configuration for Argon, Ar. Step 2: Follow the correct ordering and fill orbitals (14 – 6 = 8 eleft) 1s2 2s2 2p6 Sample Problem B Write the electron configuration for Argon, Ar. Step 2: Follow the correct ordering and fill orbitals (8 – 2 = 6 eleft) 1s2 2s2 2p6 3s2 Sample Problem B Write the electron configuration for Argon, Ar. Step 2: Follow the correct ordering and fill orbitals (6 – 6 = 0 eleft) 1s2 2s2 2p6 3s2 3p6 Sample Problem C Write the electron configuration for Bromine, Br. Sample Problem C Write the electron configuration for Bromine, Br. Step 1: Find the # of electrons (Look at the Atomic number) Br is Atomic # 35. Br has 35 e- Sample Problem C Write the electron configuration for Bromine, Br. Step 2: Follow the correct ordering and fill orbitals (35 – 2 = 33 eleft) 1s2 Sample Problem C Write the electron configuration for Bromine, Br. Step 2: Follow the correct ordering and fill orbitals (33 – 2 = 31 eleft) 1s2 2s2 Sample Problem C Write the electron configuration for Bromine, Br. Step 2: Follow the correct ordering and fill orbitals (31 – 6 = 25 eleft) 1s2 2s2 2p6 Sample Problem C Write the electron configuration for Bromine, Br. Step 2: Follow the correct ordering and fill orbitals (25 – 2 = 23 eleft) 1s2 2s2 2p6 3s2 Sample Problem C Write the electron configuration for Bromine, Br. Step 2: Follow the correct ordering and fill orbitals (23 – 6 = 17 eleft) 1s2 2s2 2p6 3s2 3p6 Sample Problem C Write the electron configuration for Bromine, Br. Step 2: Follow the correct ordering and fill orbitals (17 – 2 = 15 eleft) 1s2 2s2 2p6 3s2 3p6 4s2 Sample Problem C Write the electron configuration for Bromine, Br. Step 2: Follow the correct ordering and fill orbitals (15 – 10 = 5 eleft) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 Sample Problem C Write the electron configuration for Bromine, Br. Step 2: Follow the correct ordering and fill orbitals (5 – 5 = 0 eleft) DONE! 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 Your turn … Writing Electron Configurations #1 Writing Noble Gas Configurations A Short Cut Noble Gas Configurations Rules Find the noble gas tha appears BEFORE the element Following the usual order, write the remaining electrons contained in the element’s period Confusing…but you’ll get it. DON’T USE THE BACK OF YOUR TEXT! The ordering is incorrect and I’ll know you cheated!! =( Sample Problem C Write the noble gas configuration for Sodium. Step 1: Find the noble gas BEFORE sodium. Ne appears before Na. [Ne] Sample Problem C Write the noble gas configuration for Sodium. Step 2: Follow the ordering, starting at the period Na appears in. Na is in Period 3 [Ne] 3s Sample Problem C Write the noble gas configuration for Sodium. Step 3: Keep going until you reach Na. Only 1 step, so …. [Ne] 3s1 Sample Problem D Write the noble gas configuration for Bromine. Step 1: Find the noble gas BEFORE Br. Ar appears before Br. [Ar] Sample Problem D Write the noble gas configuration for Bromine. Step 2: Follow the ordering, starting at the period Br appears in. Br is in Period 4 [Ar] 4s Sample Problem D Write the noble gas configuration for Bromine. Step 3: Keep going until you reach Br. [Ar] 4s2 Sample Problem D Write the noble gas configuration for Bromine. Step 3: Keep going until you reach Br. [Ar] 4s2 3d10 Sample Problem D Write the noble gas configuration for Bromine. Step 3: Keep going until you reach Br. [Ar] 4s2 3d10 4p5 Sample Problem E Write the noble gas configuration for Chromium, Cr. Step 1: Find the noble gas BEFORE Cr. Ar appears before Cr. [Ar] Sample Problem E Write the noble gas configuration for Cr. Step 2: Follow the ordering, starting at the period Cr appears in. Cr is in Period 4 [Ar] 4s Sample Problem C Write the noble gas configuration for Cr. Step 3: Keep going until you reach Cr. [Ar] 4s2 Sample Problem E Write the noble gas configuration for Cr. Step 3: Keep going until you reach Cr. [Ar] 4s2 3d4 Your turn … Writing Noble Gas Configurations #1 Valence Electron Configurations The REALLY Short way. Valence Electron Configurations Rules: Do everything you did with Noble Gas configurations….. EXCEPT DON’T WRITE THE NOBLE GAS! Writing Valence Electron Configurations For example: Hydrogen, H Look at your periodic table Hydrogen is: Period 1 s block Only 1 electron Electron configurations: 1s1 Writing Valence Electron Configurations What is the valence configuration for Selenium, Se. Start at the period… 4s2 3d104p4 Your Turn … Writing Electron Configurations & Valence Configurations #1