Full Electron Configuration Lesson
advertisement

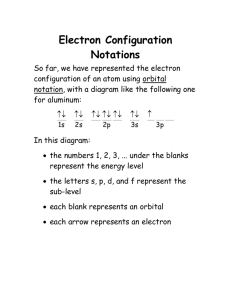
Title: Lesson 4 Full Electron Configuration Learning Objectives: • Know how to write full electron configurations using ideas of subshells BELL TIME ACTIVITY Below are examples of how you write electron configurations in HL chemistry. Try and figure out the method used to produce them. Use your periodic table. 1. 1s22s22p4 = Oxygen 2. 1s22s22s63s1 = Sodium 3. 1s22s22p63s23p64s2 = Calcium • nth energy level is divided into n sub levels • s, p, d and f identify the different sub levels • Each main level can hold a maximum of 2n2 electrons Why? Shows the existence of sub levels within an energy level. This explains the behaviour of elements. Draw out the sublevels in each main energy level. Starting at 1s, follow the arrows to give the order of the sublevels! So, the pattern for reading the electron configurations right off the periodic table is this: If you are wanting to write the electron configuration for any element, just follow this pattern and remember to stop at the element you’re representing. 1 1s2 2 2s2 2p6 3 3s2 3p6 4 4s2 3d10 4p6 5 5s2 4d10 5p6 6 6s2 5d10 6p6 7 7s2 6d10 4f14 5f14 For example, Cl (#17) which is right here on the table: So the answer would be 1s2 2s2 2p6 3s2 3p5 The short cut would be: [Ne]3s2 3p5 1 1s2 2 2s2 2p6 3 3s2 3p5 4 5 6 7 Or how about Ni (#28) 1s2 2s2 2p6 3s2 3p6 4s2 3d8 Short cut: [Ar] 4s2 3d8 1 1s2 2 2s2 2p6 3 3s2 3p6 4 4s2 3d8 5 6 7 4f14 5f14 Let’s try Bi (#83) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p3 (don’t forget the 4f14!) Short cut: [Xe]6s2 4f14 5d10 6p3 1 1s2 2 2s2 2p6 3 3s2 3p6 4 4s2 3d10 4p6 5 5s2 4d10 5p6 6 6s2 5d10 6p3 7 4f14 Abbreviated electron Configurations • Only outer electrons are shown • Inner electrons are represented as a noble gas core. • After Argon (Ar), notation can be written like: • Potassium = [Ar] 4s1 • [Ar] represents 1s22s22p63s23p6 The Uncertainty Principle • Bohr’s model assumes the electron’s trajectory can be precisely described = Not true. • Any attempted measure of an electron’s position will disturb the motion. • Focusing radiation to locate an electron will give it a ‘kick’ throwing it into a random direction. • We cannot know where an electron would be at any given time – all we can give is a probability picture of where the electron is likely to be. Atomic Orbitals By the way, the orbitals are not really little empty boxes on a line: 2p Instead, they are specific three-dimensional shapes called probability clouds that show where you are most likely to find the electron around the nucleus. The s sublevels are all spherical in shape: And they just get larger and larger as you move to higher levels 1s atomic orbital. Density of dots gives the probability of finding the electron in this region. 3s2s 1s p Orbital The p orbitals are a bit more complicated - they are peanut shaped! Within the 2p sublevel, the three orbitals are oriented at right angles to each other. They are referred to as the 2px, 2py and 2pz orbitals. And they fit together around the nucleus like this: P Orbitals Complete the Test Yourself Questions Use the ‘Sub-levels of Electrons Table’ and ‘Electron Configuration Blocks’ to help you • Page 69 • Question 11 a-e • Check your answers on page 559 BELL TIME ACTIVITY Which electronic configuration for Zn, Zinc, is correct? 1. 1s22s22s63s23p64s23d10 2. 1s22s22p63s23p64s23d10 3. 1s22s22p63s23p64s24d10