Electron Configuration
advertisement
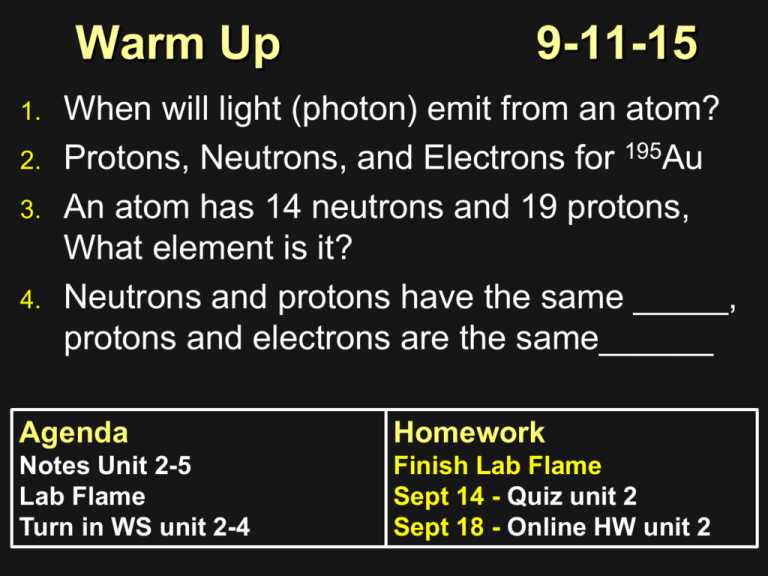
Warm Up 1. 2. 3. 4. 9-11-15 When will light (photon) emit from an atom? Protons, Neutrons, and Electrons for 195Au An atom has 14 neutrons and 19 protons, What element is it? Neutrons and protons have the same _____, protons and electrons are the same______ Agenda Homework Notes Unit 2-5 Lab Flame Turn in WS unit 2-4 Finish Lab Flame Sept 14 - Quiz unit 2 Sept 18 - Online HW unit 2 Unit 2-5 Electron Configuration (locating electrons) Energy Level • Energy level (n) – represented by a whole number • Maximum number of electrons that can fit in an energy level is: 2n2 -How many e- in level 2? -How about 3? Orbitals Orbitals (s,p,d,f) – are sublevels in each energy level S 2e P 6e D 10e Energy Level and Electron energy level (n) Sublevel per energy (n) Maximum electrons 1 s 2 2 s, p 8 3 s, p, d 18 4 s, p, d, f 32 Electron Configurations 1 2 3 4 5 6 7 Electron Configurations Electron Configurations Examples 1. 2. D orbitals are 1 less energy Cu and Cr are exceptions Electron Configuration Problems Answers Try: Ca S Ti Fe 1s22s22p63s23p64s2 1s22s22p63s23p4 1s22s22p63s23p64s23d2 1s22s22p63s23p64s23d6 Flame Test Lab Clean/Dry and Bring your plate up for the compounds (only get a small amount!!) Rinse your nichrome wire in between each compound to clean it Clean Up Procedure All solid compounds from your plate goes into the trash can Clean your lab table If your bunsen burner doesn’t have blue flame please use a wet paper towel and wipe the tip (caution hot) to get burned residue off it. Rinse your hands after you’re done cleaning up Assignments Summary Complete and turn in Unit 2-4 worksheet Finish lab write up due next class