Review 1
advertisement

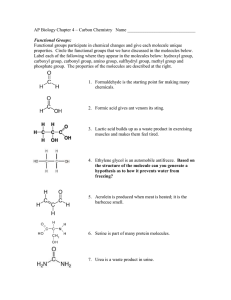
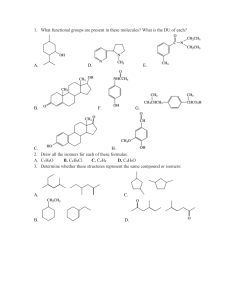
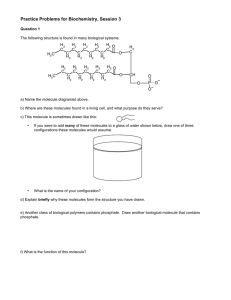
CHE 2100 Fall 2012 General Organic & Biological Chemistry Review I A general note: Short answer questions are just that, short. Writing a paragraph filled with every term you can remember from class won’t improve your answer— just answer clearly, succinctly, and in your own words. 1. Predict and label the bond angles for each Carbon in the following structure (you may redraw the structure to more clearly illustrate the angles, if you desire 2. Alkanes are also known as saturated hydrocarbons. Explain why. 3. Draw the line angle formula, condensed structural formula, and molecular formula for Ethane, labeling which is which. 4. Draw and name the two constitutional isomers of C4H10. 5. Correctly name the following compounds: 6. Draw the correct line-angle structure of 8-ethyl-2,3-dimethyldecane. 7. Circle the molecule you would expect to have the lowest melting point. Explain why. 8. What type of isomers are the following molecules to each other? Name the molecules. 9. Write the condensed structural formula of 3-nonene. 10. Name the following molecules. What type of isomers are they? 11-13. Name the following molecules: 14. Draw the structural formula for 2,2,4-trimethylpentane. What importance does this molecule have in terms of gasoline? 15. Which would you expect to have a higher boiling point: 3-methyl butane or pentane? Why? 16. Explain why cis-trans isomerization is important in vision. 17. Draw the possible products that will form when the following molecule is dehydrated in the presence of a strong acid and heat. Label the major product (if any) of the reaction. 18. Draw the possible products that will form when the following molecule is reacted with hydrofluoric acid (HF). Label the major product (if any) of the reaction.