Solids and Liquids
advertisement

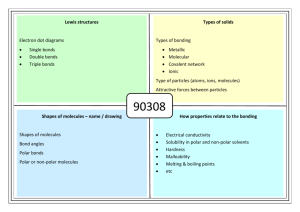
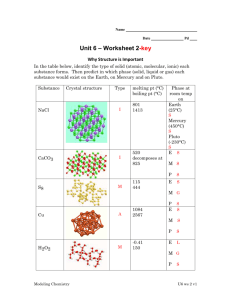
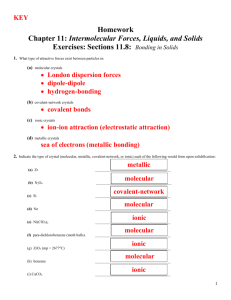
CHAPTER 14 – LIQUIDS AND SOLIDS LIQUIDS Attractive forces hold molecules close together, although molecules can still roll around 6B-1 (of 32) Solid Liquid Gas Vibrating Rolling Straight-Line PHASE CHANGES (1) EVAPORATION If surface molecules acquire enough kinetic energy to overcome the attractive forces, they can escape With liquid water in a closed container: (2) CONDENSATION Vapor molecules collide with the surface of the liquid, lose energy, and are captured When these 2 processes are equal, it looks like evaporation has stopped Really, evaporation and condensation are occurring at equal rates 6B-2 DYNAMIC EQUILIBRIUM – When 2 opposing processes in the same system proceed at equal rates Evaporation: liquid → vapor Condensation: vapor → liquid Equilibrium: liquid ↔ vapor EQUILIBRIUM VAPOR PRESSURE – The pressure exerted by a vapor when it is in equilibrium with its liquid 6B-3 EVP depends on temperature Temp (ºC) EVP (torr) 10 9 20 30 6B-4 18 32 BOILING Atmospheric Pressure A bubble is full of steam that exerts water’s EVP A bubble survives when its EVP equals atmospheric pressure When bubbles can reach the top the liquid is boiling BOILING POINT – The temperature at which the EVP of the liquid equals the prevailing atmospheric pressure 6B-5 Temp (ºC) 5 10 20 30 95 100 122 6B-6 EVP (torr) 7 9 18 32 634 760 1557 HEATING CURVES All pure substances can exist as solid, liquid, or gas NORMAL MELTING POINT – At 1 atm pressure, the temperature a solid turns into a liquid (also called the NORMAL FREEZING POINT) NORMAL BOILING POINT – At 1 atm pressure, the temperature a liquid turns into a gas 6B-7 HEAT OF FUSION – The heat needed to melt a specific amount of a solid For ice: 334 J/g or 6.02 kJ/mol HEAT OF VAPORIOZATION – The heat needed to boil a specific amount of a liquid For water: 6B-8 2,250 J/g or 40.6 kJ/mol Calculate the heat energy needed to change 10.00 g of ice at 0.0ºC to steam at 100.0ºC. Melting: Liquid: Boiling: (334 J/g)(10.00 g) = 3,340 J (4.184 J/gCº)(10.00 g)(100.0 Cº) = 4,184 J (2,250 J/g)(10.00 g) = 22,500 J 30,024 J = 30,000 J 6B-9 SUBLIMATION – A solid changing directly to a gas Iodine (I2), Dry Ice (CO2), Mothballs (C10H8) DEPOSITION – A gas changing directly to a solid 6B-10 SOLIDS CRYSTALLINE SOLID – A solid with a definite particle arrangement AMORPHOUS SOLID – A solid with a random particle arrangement Sand Crystalline 6B-11 Glass Amorphous MELTING POINTS OF SOLIDS Melting points (and boiling points) depend on the attractive forces BETWEEN particles (molecules, atoms, or ions) TYPES OF SOLIDS (1) Molecular (A) Nonpolar (B) Polar (2) Atomic (A) Nonmetallic Network (B) Metallic Network (3) Ionic 6B-12 (1) MOLECULAR SOLIDS INTRAMOLECULAR FORCES – Forces WITHIN a molecule Covalent bonds They determine how reactive a molecule is INTERMOLECULAR FORCES – Forces BETWEEN molecules They determine melting points and boiling points Intramolecular Forces Intermolecular Forces 6B-13 There are two types of molecular solids (A) NONPOLAR MOLECULAR Made of individual nonpolar molecules Examples: O2, CO2 (linear) 6B-14 Intermolecular Forces: LONDON DISPERSION FORCES (or VAN DER WAALS FORCES) – The attraction of electrons in one molecule to the nuclei in a neighboring molecule due to the synchronizing of electrons δ+ δ- δ+ δ- LDF’s are weak attractive forces, so nonpolar molecular solids have low melting points LDF’s Increase in strength as the number of electrons per molecule increase 6B-15 Although they are composed of atoms, Noble Gases behave as nonpolar molecular matter 6B-16 (B) POLAR MOLECULAR Made of individual polar molecules Examples: HCl, SO2 (bent) 6B-17 Intermolecular Forces: London Dispersion Forces, and DIPOLE-DIPOLE ATTRACTIONS – The attraction of the positive end of one polar molecule to the negative end of another δδ+ δ+ δ- D-D Attractions are weak, so polar molecular solids have low melting points D-D Attractions are stronger than LDF’s, so polar molecular solids have higher melting points than nonpolar molecular solids 6B-18 Polar molecules that have a H bonded to either N, O, or F have a third type of attractive force HYDROGEN BONDING – An intermolecular attraction between a H in one molecule and a N, O, or F in a neighboring molecule Hydrogen Bonding Polar molecules that can H-Bond have higher melting points than polar molecular that cannot 6B-19 H-Bonding: Causes ice to be less dense than water by forming an open crystal lattice produces the folded shapes of proteins holds together the strands of DNA molecules 6B-20 (2) ATOMIC SOLIDS There are two types of atomic solids (A) NONMETALLIC NETWORK Made of millions of nonmetal atoms Examples: Cx (diamond), (SiO2)x (sand) Attractive forces: Covalent bonds 6B-21 All atoms are covalently bonded together, so the entire crystal is like one giant molecule Covalent Bonds are strong, so nonmetallic networks have high melting points 6B-22 (B) METALLIC NETWORK Made of millions of metal atoms Examples: Fe, Au, bronze (Cu + Sn), brass (Cu + Zn) Attractive forces: Metallic Bonds 6B-23 METALLIC BONDS – The attraction between metal nuclei and the valence electrons of the millions of atoms Metallic Bonds are strong, so metallic networks have high melting points 6B-24 ALLOY – A solid solution of metals SUBSTITUTIONAL ALLOY Brass 6B-25 INTERSTITIAL ALLOY Steel (3) IONIC SOLIDS Made of millions of positive and negative ions Examples: NaCl, CuSO4 Attractive forces: Ionic Bonds 6B-26 + - + + - + - Ionic Bonds are strong, so ionic substances have high melting points 6B-27 Substance MP (ºC) Substance Type Attractive Forces H2 O2 Ar -259 -218 -189 Nonpolar Molecular “ “ LDF “ “ HCl HBr -114 -89 Polar Molecular “ LDF, D-D “ NH3 H2O -78 0 “ “ LDF, D-D, H-B “ NaCl MgF2 801 1266 Ionic “ Ionic Bonds “ Fe W 1535 3410 Metallic Network “ Metallic Bonds “ (SiO2)x Cx 1723 3550 Nonmetallic Network “ Covalent Bonds “ 6B-28 ELECTRICAL CONDUCTION ELECTRICIAL CONDUCTION – The movement of charges particles (electrons or ions) through a material Conductors: Metallic networks Electrons can move throughout the metallic bonds Nonconductors: Molecular solids or Nonmetallic networks Electrons are locked in covalent bonds Ionic solids Ions are locked in position as a solid, BUT melted or dissolved in water, the ions can move and the ionic material conducts 6B-30 REVIEW FOR TEST Pressure (mm Hg, torr, atm) Temperature (ºC, K) Standard Temperature and Pressure (STP) Boyle’s Law, Charles’ Law, Avogadro’s Law Char-Boyled Law Ideal Gas Law Partial Pressure Water Vapor Pressure Pressure of Gases Collected in Lab Over Mercury Over Water Gas Volumes in Chemical Reactions 6B-31 REVIEW FOR TEST Dynamic Equilibrium Melting, Boiling, Sublimation Heat of Fusion, Heat of Vaporization, Specific Heat Capacity Heating Curves, Heat Calculations Crystalline, Amorphous Solids Intramolecular Forces, Intermolecular Forces London Dispersion Forces, Dipole-Dipole Interactions, Hydrogen Bonding Attractive Forces, MP’s, Conductivity of Molecular Solids (Nonpolar Molecular and Polar Molecular) Atomic Solids (Nonmetallic Networks and Metallic Networks) Ionic Solids Alloys (Substitutional, Interstitial) 6B-32