Metallic Bonds
advertisement

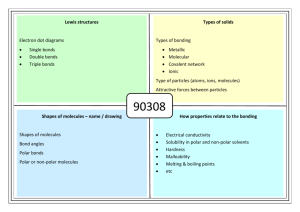
Bonding Ionic – Metal/Non-metal Cation/Anion Cation: low ionization energy, lose electrons Anion: high ionization energy, gain electrons Lattice energy – Energy required to separate a mole of solid ionic compound into its ions(energy released when ionic compound formed.) L.E.=Q1Q2/r Smaller radius-larger lattice energy Higher charges-larger lattice energy Covalent – Non-metals Both have attraction for electronsorbitals overlap and electrons “share” Polar: Uneven sharing – H2O, HCl Dipole – slightly positive end and slightly negative end Larger difference in electronegativity means larger dipole- more polar Non-polar: Even sharing – CO2, CH4, PH5, N2 Metallic Bonds – In metals Free floating valence electrons that surround positively charged metal ions. Electrons are free to flow – conduct electricity Dot Structures: (Know) O2, CO2, N2, F2, H2, CN-, C2H2, C2H4 Resonance Structures: SO3 Measured bond lengths between S and O are actually somewhere in between length of a single and double bond. Bond Length Single bond-longer and weaker Triple bond-shorter and stronger Hybridization -mixing atomic orbitals to form molecular orbitals of equal energies. Sigma + Pi Bonds CH3COCHClCN Intermolecular Forces –Between molecules Ionic Hydrogen bonding H- F, O, N Dipole-Dipole Polar London Dispersion All types larger molecule = more London forces -London dispersion-Brief instants when electron density greater on one side of atom than the other. - Temporary polarity (POLARIZABLE) Stronger IMF… B.P? Surface tension? Viscosity? ΔHvap? Vapor pressure? Explain the Trend: Compound CH4 NH3 H2 O BP (˚C) -162 -33 100 Shapes/ Bond Angles Vapor pressure (top of liquid) Boiling Vapor pressure is equal or greater than the atmospheric pressure. How can we make water boil at temps less than 100˚C High altitudes – water boils at lower temperature (it takes longer to cook at high altitude) Pressure cooker - Boils at higher temps. Molecular solids – Solids held together by IMFs – NOT bonds Ionic solids – Solids held together by ionic bonds – salts Covalent network solids – Covalent bonded – large structures graphite, diamond, quartz Metallic solids – arranged in regularly packed structures