Chapter 5
advertisement

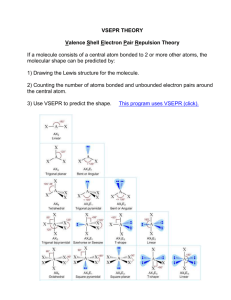
Chapter 5 Chemical Bonds Some Definitions • Valence electrons • Octet Rule • Ion – Cation – Anion • Electronegativity • Polarity Ions on the Periodic Table CATION +1 +2 CATION Non-Ionic ANION +3 ? -3 -2 -1 ? Electron-dot structures • • • • Also called Lewis Dot Structures Represent an element’s valence electrons Representative of the valence electrons for the entire group Features: 1. Each electron is represented by a dot surrounding the atomic symbol of an element 2. Dots are only allowed in 4 positions (top, bottom, left, right), no in-betweens Electron-dot structures • What is the electron-dot structure for sodium? • What is the electron-dot structure for oxygen? • What is the electron-dot structure for neon? Practice • Write the electron-dot symbols for a sodium metal atom and a chlorine atom, and their ions. Na + Na Cl Cl Ionic Bonding Sodium Chloride Ionic Bonding Atoms shuffle electrons, then are attracted to each other because of net charge = IONIC BOND Formulas of Ionic Bonding • Textual way of describing what is physically happening • Ions come first, then attraction of charges = BALANCE Writing Ionic Formulas Write the formula for calcium fluoride. • Write the symbols for the ions, and their charges. • Cross over the charge numbers so they become subscripts. • Rewrite the formula, dropping the charge superscripts. 2+ Ca 1 FCaF 2 2 1- Naming Ionic Compounds • Between 2 Elements 1. Name of metal ion 2. Name of non-metal ion, –ide suffix Those Pesky Transition Metals • • Chromium Cr2+ chromium(II) 3+ Cr chromium(III) Variable valence =+ we cannot predict ionic Copper Cu copper(I) charge from the Cu group number 2+ copper(II) iron(II) Roman Iron numeral Fe in3+2+parenthesis is used to Fe iron (III) indicateManganese ion Mn2+ manganese(II) Mn3+ manganese(III) Nickel Ni2+ nickel(II) Ni3+ nickel(III) Zinc Zn2+ zinc(II) Naming Ionic Compounds Write the name for ZnCl2 1. Cross over the subscripts so they become charges • Remember that the metal (positive ion) is always first. 2. Write the name of each element and add – ide ending to the non-metal 3. Be sure to include a roman numeral in parenthesis if the metal is a transition element Practice • React lithium and sulfur together. What will happen? Li + Li Stable? S - 2- S + Li 2Li+ + S2- = Li2S Bond, Chemical Bond • Ionic Bonding 1. Electron transfer from metal to non-metal 2. The more electronegative atom wins! • Covalent Bonding 1. Electron sharing between non-metals 2. Atoms of similar electronegativity Covalent Bonding • 2 Atoms share valence electrons to create stable bonds • No transference of electrons • Between atoms of similar electronegativity • Non-metals • Atoms seeking to fill an octet: achieve the next highest noble gas configuration Naming Covalent Compounds • First non-metal = elemental name • Second non-metal = elemental name with –ide ending – E.g. oxide, sulfide, bromide • Subscripts = use prefixes to name multiple atoms • Exception: for oxygen, drop the “a” at the end of the prefixes – E.g. tetroxide, hexoxide, pentoxide Example • Name the following covalent compound N2O3 dinitrogen trioxide C Cl4 * carbon tetrachloride * By convention, the mono- on the first atom is not generally included Example • Write the formula for the following compound: tetraphosphorus hexoxide 4 P’s 6 O’s P4O6 Electron-dot Formulas 1. Determine the arrangement of atoms • For “central” atom(s), it will generally be single, surrounded by multiple other atoms 2. Determine the total number of valence electrons 3. Attach the central atom to each bonded atom by an electron pair • Represented by a dash 4. Arrange the remaining electrons around the outer elements first, then the central atom 5. If octets are not complete, form a multiple bond Practice • Draw the electron-dot formula for nitrogen tribromide No multiple bonds necessary! Br N Br Br Practice • Draw the electron-dot formula for carbon dioxide. Multiple bonds necessary! O C O Diatomic Molecules • Several non-metals are naturally diatomic: more stable electron configuration • Oxygen and nitrogen form multiple bonds to achieve stability N N N N Polyatomic Ions • A group of atoms with a net electrical charge Most are non-metals covalently bonded to oxygen Net charge: -1, -2, -3 shared among the atoms Two notable exceptions are +1: 1. Ammonium (NH4+) 2. Hydronium (H30+) Formed when a larger molecule splits unevenly Names of Polyatomic Ions • Memorize the common –ate ions • • • • • • Nitrate Chlorate Bromate Carbonate Sulfate Phosphate NO3ClO3BrO3CO32SO42PO43- Remember these exceptions! hydroxide OHammonium NH4+ hydronium H3O+ – Add an O = per- prefix on –ate ion (e.g. perchlorate, ClO4-) – Lose an O = -ate changes to –ite (e.g. chlorite, ClO2-) – Lose another O = hypo- prefix on –ite ion (e.g. hypochlorite, ClO-) • All forms (-ate, -ite, per- hypo-) of an ion have the same charge! Naming Polyatomic Ions • Recognize the polyatomic ion as a UNIT • Name the metal ion first, then the polyatomic ion • No extra prefixes or suffixes CaCO3 Calcium Carbonate VSEPR Models • Based on several assumptions: 1. Atoms in a molecule are bound together by at least one pair of electrons: a bonding pair 2. Some atoms may possess unbound, or lone pair, electrons 3. Arrangements of electrons in the molecule will minimize electron interactions 4. Lone pairs occupy more space than bonded pairs 5. Double bonds occupy more space than single bonds 6. Multiple bonds are considered as single bonds when determining molecule shape VSEPR Models • Electron group geometry vs. Molecular geometry (shape) – – • • Electron group geometry = placement of electrons around the central atom Molecular geometry = 3-dimensional arrangement of the molecule Electron group geometry determines shape Shape only refers to atoms and bonded electron groups, not lone pairs Points to Remember 1. ONLY the number of electron groups that surround the central atom are considered in determining molecular shape 2. 1 electron group may consist of a triple bond, a double bond, a single bond, or a lone pair 3. A lone pair will give a different molecular shape than a single, double, or triple-bonded atom at the same position VSEPR Models Electron Pairs Bonded Pairs Lone Pairs Molecular Geometry Examples 2 2 0 Linear CO2 O =C =O CO2 Source: http://www.molecules.org/VSEPR_table.html VSEPR Models Electron Pairs Bonded Pairs Lone Pairs Molecular Geometry Example 3 2 1 Bent 120° NO2- N O NO2 Source: http://www.molecules.org/VSEPR_table.html - O VSEPR Models Electron Pairs Bonded Pairs Lone Pairs Molecular Geometry Example 3 3 0 Triangular (Planar) NO3CO32- O N NO3Source: http://www.molecules.org/VSEPR_table.html O O VSEPR Models Electron Pairs Bonded Pairs Lone Pairs Molecular Geometry Example 4 2 2 Bent 109.5° H2O H H O H2O Source: http://www.molecules.org/VSEPR_table.html VSEPR Models Electron Pairs Bonded Pairs Lone Pairs Molecular Geometry Example 4 3 1 Pyramidal NH3 H NH3 Source: http://www.molecules.org/VSEPR_table.html N H H VSEPR Models Electron Pairs Bonded Pairs Lone Pairs Molecular Geometry Example 4 4 0 Tetrahedral CH4 H H CH4 Source: http://www.molecules.org/VSEPR_table.html C H H Electronegative Atom Monster Electronegativity increases Predicting Bond Type Source: http://www.chem.ufl.edu/~chm2040/Notes/Chapter_11/writing.html Polarity • Is a BOND polar or non-polar? • Is a MOLECULE polar or non-polar? • Be careful! Polar bonds can make a nonpolar molecule! Bond = non-polar Cl — Cl Molecule = non-polar Polarity • Is a BOND polar or non-polar? • Is a MOLECULE polar or non-polar? • Be careful! Polar bonds can make a nonpolar molecule! Opposing dipoles cancel! Bonds = polar O=C=O Molecule = non-polar Polarity • Is a BOND polar or non-polar? • Is a MOLECULE polar or non-polar? • Be careful! Polar bonds can make a nonpolar molecule! Dipoles do not cancel! Bonds = polar H O Molecule = polar H Between Molecules: Hydrogen Bonds • Hydrogen attached to highly electronegative atoms F, O, N – Strong dipoles • Attraction of opposite dipoles http://images.google.com/imgres?imgurl=http://polymer.bu.edu/~fstarr/net.jpg&imgrefurl=http://polymer.bu.edu/~fstarr/water.ht ml&h=248&w=350&sz=17&tbnid=4GT8aTZv8MkJ:&tbnh=82&tbnw =116&hl=en&start=49&prev=/images%3Fq%3Dhydrogen%2Bbond%26start%3D40%26svnum%3D10%26hl%3Den%26lr%3D%26rls%3DGGLC,GGLC:1969 -53,GGLC:en%26sa%3DN How Plants Work Source: http://www.agridept.gov.lk/Techinformations/Hponics/images/P_24.jpg Source:http://upload.wikimedia.org/wikipedia/en/thumb/c/c8/Coastredwood.jpg/250px-Coastredwood.jpg Hydrogen Bonding at our Core Source:http://colossus.chem.umass.edu/chandler/ch112/dna_hbond.gif Between Molecules: Dipole-Dipole • Different nonmetals bonded together • Partial charges: Dipoles • Attraction of opposite dipoles http://images.google.com/imgres?imgurl=http://library.tedankara.k12.tr/chemistry/vol2/dipole-dipole%2520forces/z105.jpg&imgrefurl=http://library.tedankara.k12.tr/chemistry/vol2/dipoledipole%2520forces/z105.htm&h=570&w=409&sz=65&tbnid=ljWYJbTg9PEJ:&tbnh=131&tbnw=93&hl=en&start=2&prev=/images%3Fq%3Ddipoledipole%26svnum%3D10%26hl%3Den%26lr%3D%26rls%3DGGLC,GGLC:1969-53,GGLC:en Between Molecules: Dispersion • Non-polar molecules • Temporary dipoles because of nonuniform electron cloud (not dispersed evenly) • Weak bonds Source: http://touregypt.net/vdc/slide.86.jpg To Sum Up the Forces… Practice • For your molecule, determine: – Name – Electron-dot structure – 3-dimensional structure – VSEPR shape – Intramolecular force – Intermolecular force