VSEPR - Valence Shell Electron Pair Repulsion Theory
advertisement

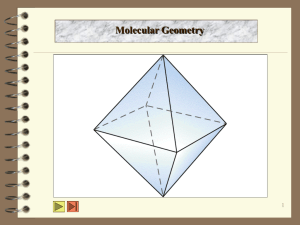
VSEPR - Valence Shell Electron Pair Repulsion Theory •is used to predict the shape of molecules. •involves valence shell e- pairs. •is based on the premise that e- repel each other, and thus e- pairs will get as far apart as possible in 3-D space. •can also be used to predict structures of molecules or ions that contain multiple bonds or unpaired electrons. •does fail in some cases. # e Example Electron Dot Diagram 3-D Diagram 2 3 4 5 6 When determining shapes using VSEPR theory, multiple bonds are treated like single bonds. 1)Predict the shapes of CaH2, AsF5, SeCl6 2) Draw electron dot diagrams for: NH3, H2S, Cl2O i) How many pairs of electrons are around the central atom? ii) How many are bonding? iii) Determine the polarity of each bond.