SCH4U1_02_07a VSEPR Note and Examples
advertisement

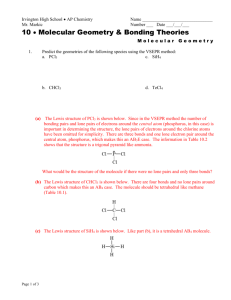
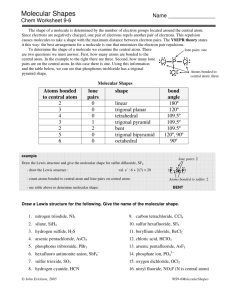
VSEPR THEORY Valence Shell Electron Pair Repulsion Theory If a molecule consists of a central atom bonded to 2 or more other atoms, the molecular shape can be predicted by: 1) Drawing the Lewis structure for the molecule. 2) Counting the number of atoms bonded and unbounded electron pairs around the central atom. 3) Use VSEPR to predict the shape. This program uses VSEPR (click). Examples of how to use VSEPR: e.g. 1) PF5 1. Draw the Lewis structure. 2. Bonded atoms = 5 Lone pairs = 0 3) trigonal bipyramidal shape (sp3d hybridization) e.g. 2) SO42- (sulfate ion) 1. Draw the Lewis structure (slightly tricky, don’t forget the 2 extra electrons!) 2. Bonded atoms = 4 Lone pairs = 0 3) tetrahedral shape (sp3 hybridization + 2 π bonds) e.g. 3) SF4 1) Draw the Lewis structure. 2) Bonded atoms = 4 Lone pairs = 1 3) see-saw molecular shape (sp3d hybridization, trigonal bipyramidal electron arrangement) e.g. 4) BrF5 1) Draw the Lewis structure 2) Bonded atoms = 5 Lone pairs = 1 3) square pyramidal shape (sp3d2 hybridization, octahedral electron configuration)