ppt file
advertisement

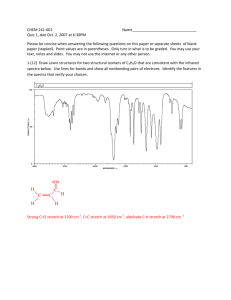
Lecture 11 IR Theory Next Class: • Lecture Problem 4 due • Thin-Layer Chromatography This Week In Lab: Ch 6: Procedures 2 & 3 Procedure 4 (outside of lab) Next Week in Lab: • Ch 7: PreLab Due • Quiz 4 • Ch 5 Final Report Due Spectroscopy NMR (Nuclear Magnetic Resonance Spectroscopy): •Uses radio waves (electromagnetic radiation) •Interacts with sample’s nuclei in the presence of a magnet •Effect: nuclei flip and relax (known as resonance) 1H NMR: Determine bond connectivities/pieces of a structure, whole structure IR (Infrared Spectroscopy) •IR radiation •Interacts with molecule as a whole •Effect: bond vibrations within molecule IR Use: Determine the functional groups present in a structure: -OH, C=O, C-O, NH2, C=C, CC, C=N, CN An IR Spectrum of Hexanol IR Spectroscopy Main Use: To detect the presence or absence of a functional group (specific bonds) in a molecule How It Works: 1. Bonds vibrate freely at specific wavelengths (wavenumbers) 2. Want to cause the bonds to increase the magnitude of this vibrational frequency 3. Subject compound to IR radiation, 4000-625 cm-1 cm-1 is the unit for wavenumber (n) (The numbers of waves within 1 cm) n is directly proportional to energy (unlike wavelength) 4. Bonds absorb energy equal to their natural vibrational energy - it is quantized. This absorption of energy causes a change in dipole moment for the bond. 5. Upon absorption, bonds stretch and/or bend; the IR measures this absorption. Vibrational Modes of Bonds Correlation Chart Specific bonds absorb specific IR radiation and signals will appear within certain wavenumber ranges (similar to NMR). Your lab notebook also has an IR correlation chart. Correlation Chart Specific bonds absorb specific IR radiation and signals will appear within certain wavenumber ranges (similar to NMR). Correlation of Bond St retching and IR A bsorption (See also Correlation Ch art & Table in Lab Gu ide) Wavenumber Range (cm-1) Type of Bond Group Family of Compounds Single Bonds —C— H Alkanes 2850-3300 =C— H Alkenes, aromatics 3000-3100 C—H Alkynes 3300-3320 O—H Alcohols 3200-3600 N—H Amines 3300-3500 C—O Ethers, Esters, Alcohols Carboxylic Acids 1330-1000 C=C Alkenes, aromatics 1600-1680 C=O Carbonyls 1680-1750 Aldehydes, ketones 1710-1750 Carboxylic acids 1700-1725 Esters, amides 1680-1750 C=N Imines 1500-1650 CC Alkynes 2100-2200 CN Nitriles 2200-2300 Double Bonds Triple Bonds IR spectrum of hexanoic acid O OH Funct ional group r egion: 155 0-4 000 cm- 1 Most use ful porti on o f IR spect rum Evaluat e t his p orti on fo r your spect ra l unknown Fingerpr int reg ion: 40 0- 1550 cm- 1 More diff icult to i nte r pret ; may cont ain useful i nf or mati on Different bond stretches & what their signals look like in IR: A: O-H stretch (strong, broad) C: C-H stretch (strong, sharp) E: CC or CN stretch (sharp) F: C=O stretch (strong, medium to sharp) G: C=C stretch (sharp) J: C-O stretch (strong, medium) K: C-X stretch (sharp) Lab Guide Problem 16.1 A sample is known to have the molecular formula C4H10O and to be one of two constitutional isomers, either t-butyl alcohol or isopropyl methyl ether. What is the structure of the unknown? QuickTime™ and a TIFF (LZW) decompressor are needed to see this picture. Lab Guide Problem 16.2(a) Indicate how the following pairs of compounds could be distinguished using characteristic IR peaks: (a) Benzaldehyde (C6H5O) and benzoic acid (C6H5COOH) 1. Consider each structure: 2. Determine the main differences that would be seen in IR. Lab Guide Problem 21.20 An unknown oxygen-containing compound is suspected of being an alcohol, a ketone, or a carboxylic acid. Its IR spectrum shows a broad strong peak at 3100-3400 cm-1 and a strong, sharp peak at 1700 cm-1. What kind of compound is it? Consider what type of bonds appear in the ranges given. Refer to correlation chart. Broad peak at 3100-3400 cm-1 Strong, sharp peak at 1700 cm-1 Lab Guide Problem 21.21 A compound with molecular formula C4H8O shows no absorption in the IR region near 1700 cm-1 or 3400 cm-1. What can you deduce about its structure? Propose one possible structure. What can you deduce about its structure? Propose one possible structure. 1. Calculate HDI. 2. Give possible structure(s):