EXAM L 9a. Practical Infrared Explain the wavenumber shift in C≡C
advertisement

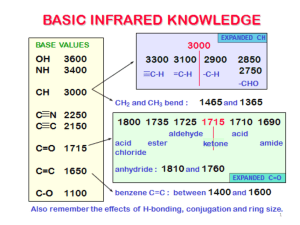
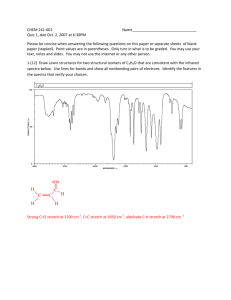
EXAM L 9a. Practical Infrared Explain the wavenumber shift in C≡C, C=C and C-C bonds. Use the following equation: 1 k 2 The bands are in decreasing energy order in a way like this C≡C, C=C and C-C. The reuced mass is the same for all the three chemical groups, only the k value difference can cause the wavenumber shift. The C≡C frequency is the highest about 2100 cm-1, the strongest bond having the highest k value. The propane has four characteristic C-H band about 2800 cm-1. What is the sequence of the bands in energy? The energy decreases as the wavenumber decreases. The order of vibrations in this broad band is as follows: asymmetric stretching methyl, symmetric stretching methyl, asymmetric stretching methylene, symmetric stretching methylene. The C≡C stretch band at 2119 cm-1 in 1-octyne disappears in 4-octyne, explain this experimental fact. Compare bands C≡C stretch at 2119 cm-1 and C≡N stretch at 2249 cm-1. The latter band is more intense than the C≡C. Explain this experimental fact. Hint: polarity The two characteristic spectral range of carboxylic acids. Compare the number of peaks arising from N-H vibrations in molecules containing primary, secondary and tertiary amin group. Primary amines have two N-H bonds, therefore they typically show two spikes that make this band. Secondary amines have only one N-H bond, which makes them show only one spike. Finally, tertiary amines have no N-H bonds, and therefore this band is absent from the IR spectrum altogether.