17 Analytical Techniques
advertisement

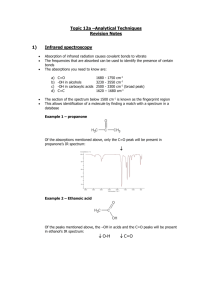
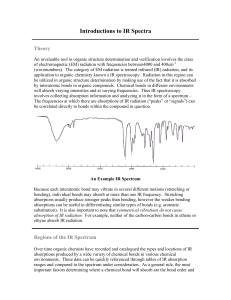
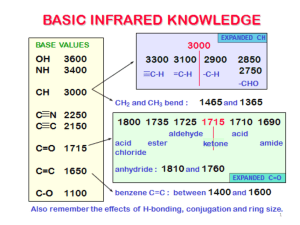
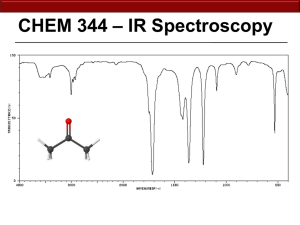
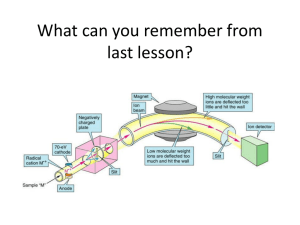
17.1 Mass Spectrometry Learning Objectives: 1. Describe how mass spectrometry can be used to identify unknown compounds. Used to Find Molecular Mass of Organic Compounds 1. Compound enters MS as gas. 2. Electron gun ionises sample. 3. Positive ions accelerated through MS as beam of ionised molecules. 4. Ions deflected by magnetic field into a detector. 5. Strength of the magnetic field related to m/z (m/z = mass as charge = +1). 6. Detector measures abundance. Mass Spectrum Why are there so many peaks? • molecular ion = ion formed from whole molecule • The main peak furthest to the right corresponds to the mass of the molecular ion. • Fragmentation = molecule breaks up during the MS process to form smaller ions • The pattern can then be used as a fingerprint to identify the molecule. Mass Spectrum of Pentane High Resolution MS • MS usually to nearest whole number. • Can be difficult to differentiate between different molecules with same similar Mr. • High res MS can measure masses to three or four decimal places. • More accurate, can be used to differentiate different molecules of similar masses. 17.2 Intra-Red Spectroscopy Learning Objectives: 1. Describe IR spectroscopy. 2. Be able to interpret IR spectra. All bonds are vibrating • Each bond has a certain frequency of vibration. • This frequency corresponds with certain frequencies (wavelengths) of IR radiation. • Bonds can absorb IR radiation to vibrate more. • The frequencies which are absorbed can be detected and used to identify what bonds are in a molecule. Infra-Red Spectrometer 1. Beam of IR radiation with range of frequencies passed through a sample. 2. The sample absorbs certain frequencies. 3. Instrument plots the transmittance (intensity of frequencies which make it through the sample). 4. The absorbed frequencies will be “missing” from the chart. IR Spectrum Peaks Correspond to Specific Bonds FINGERPRINT REGION • • • • organic molecules have a lot of C-C and C-H bonds within their structure spectra obtained will have peaks in the 1400 cm-1 to 800 cm-1 range this is referred to as the “fingerprint” region the pattern obtained is characteristic of a particular compound the frequency of any absorption is also affected by adjoining atoms or groups. IR SPECTRUM OF A CARBONYL COMPOUND • carbonyl compounds show a sharp, strong absorption between 1700 and 1760 cm-1 • this is due to the presence of the C=O bond IR SPECTRUM OF AN ALCOHOL • alcohols show a broad absorption between 3200 and 3600 cm-1 • this is due to the presence of the O-H bond IR SPECTRUM OF A CARBOXYLIC ACID • • • • carboxylic acids show a broad absorption between 3200 and 3600 cm-1 this is due to the presence of the O-H bond they also show a strong absorption around 1700 cm-1 this is due to the presence of the C=O bond IR SPECTRUM OF AN ESTER • esters show a strong absorption between 1750 cm-1 and 1730 cm-1 • this is due to the presence of the C=O bond WHAT IS IT! One can tell the difference between alcohols, aldehydes and carboxylic acids by comparison of their spectra. O-H STRETCH ALCOHOL C=O STRETCH ALDEHYDE O-H STRETCH CARBOXYLIC ACID AND C=O STRETCH