Infrared Spectroscopy
advertisement
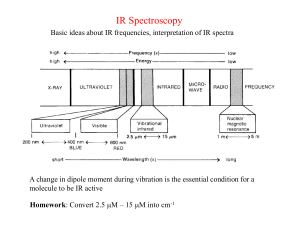
INFRARED SPECTROSCOPY Electromagnetic Spectrum Bond Stretching High Frequency Stretching Low Frequency Bending Frequency of Absorption Measured in cm-1 (Wavenumbers) Wavelength units typically microns ( m) 10,000 m = 1 cm or 1m = 10 -4 cm Frequency units typically measured in cm-1 = # waves or cycles in 1 cm (wavenumbers) Mid-Infrared frequency range is 4000 - 400 cm-1 (2.5 - 25 m) High Frequency to Low Frequency Functional Groups Region 4000 - 1400 cm-1 Fingerprint Region 1400-600 cm-1 General Trends: i) Stretching frequencies are higher than corresponding bending frequencies. (It is easier to bend a bond than to stretch or compress it.) ii) Bonds to hydrogen have higher stretching frequencies than those to heavier atoms. iii) Triple bonds have higher stretching frequencies than corresponding double bonds, which in turn have higher frequencies than single bonds. (Except for bonds to hydrogen). IR Spectrum of Octane Decane 2,2,4-Trimethylpentane Hybridization of Carbon Affects n of Absorption Hexane and Hexene 1-Pentene 1-Dodecene 2-Methyl-1,3-butadiene (Isoprene) Terminal Alkynes are Readily Identifiable with IR 1-Heptyne Aromatics • C-H str (sp2) at 3050-3100 cm-1 • C-C str (breathing) 1600 & 1500 cm-1 H Toluene o-Xylene Alcohols O-H str at 3600-3200 cm-1 C-O str at 1050-1150 cm-1 Cyclohexanol O-H Stretch 2-Methyl-1-butanol Benzyl alcohol o 1 Amines 2-Methylpentanediamine 2o Amine Dipropylamine Carbonyl Compounds Acetone Cycloheptanone Acetophenone Conjugation Lowers n of Absorption Ketone and Aldehyde Octanal Carboxylic Acid Hexanoic Acid Phenyl Acetate, an Ester Ethyl Butanoate Methyl Benzoate Benzoic Anhydride, an Acid Anhydride Butanamide, an Amide O O CH3CH2OCCH3 O CH2CH C N CH2=CHCH2CH2CH3 HC CCH2CH2OH CH3CH2CH2NH2 CH2CH2CH2CH2CO2H