Name: Date: ______ Period: ______ Page#: _____ CHAPTER 6
advertisement
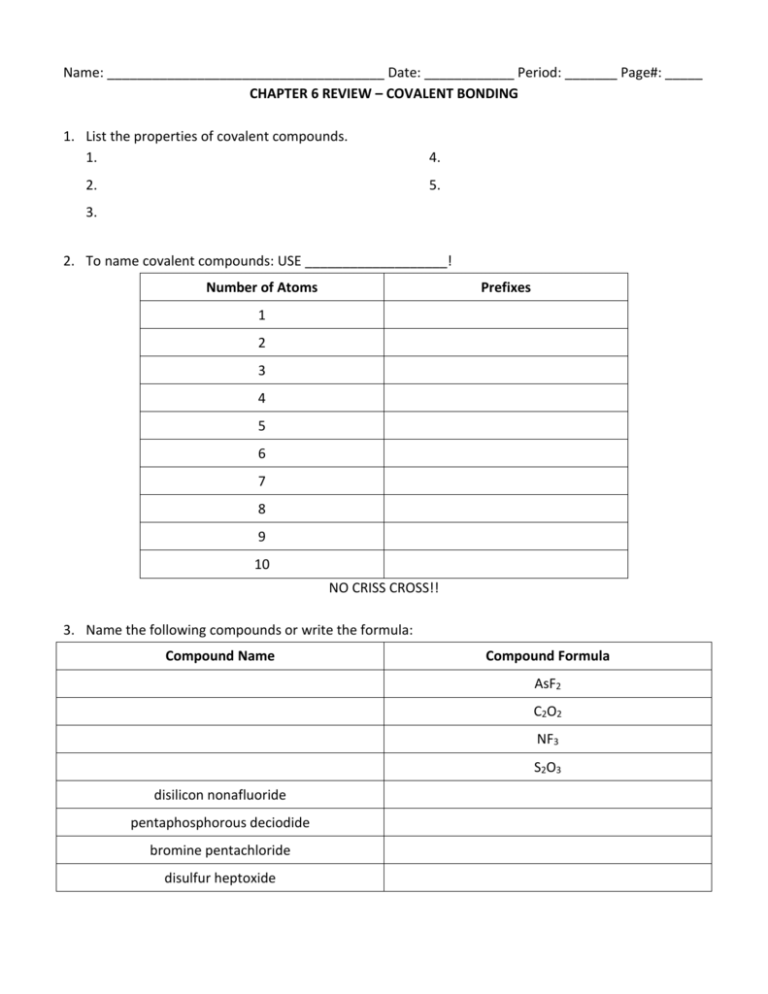
Name: _____________________________________ Date: ____________ Period: _______ Page#: _____ CHAPTER 6 REVIEW – COVALENT BONDING 1. List the properties of covalent compounds. 1. 2. 4. 5. 3. 2. To name covalent compounds: USE ___________________! Number of Atoms Prefixes 1 2 3 4 5 6 7 8 9 10 NO CRISS CROSS!! 3. Name the following compounds or write the formula: Compound Name Compound Formula AsF2 C2O2 NF3 S2O3 disilicon nonafluoride pentaphosphorous deciodide bromine pentachloride disulfur heptoxide 4. Identify the shared pair and lone pairs in the diagram below: How many valence electrons are shown in this structure? _______ 5. Draw an example of a compound with a: Single Bond Double Bond Triple Bond 6. Draw the Lewis Structures for: CH4 NF3 H2O CO2 SiO2 SF2 7. Describe the amount of transfer of electrons for: An ionic bond: A polar covalent bond: A nonpolar covalent bond: 8. Identify the type of bond that exists with these electronegativity differences: 0 – 0.3 0.4 – 1.6 1.7 9. Using the table of electronegativities, identify the bond type between the following elements: Electronegativity Elements Element 1 Element 2 Electronegativity Difference Bond Type Ca to F N to O Se to I Fe to C 10. What is the difference between intermolecular and intramolecular forces? 11. Define each type of intermolecular force. Then draw a picture of what this force looks like. Type of Force: Defined Hydrogen Bonding: Picture Dipole-Dipole: Dipole-Induced Dipole: London Dispersion Forces: 12. List all the properties of liquids that are affected by intermolecular forces.