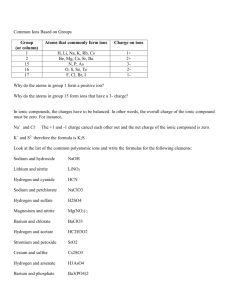
pH - Melanie Bennett
advertisement
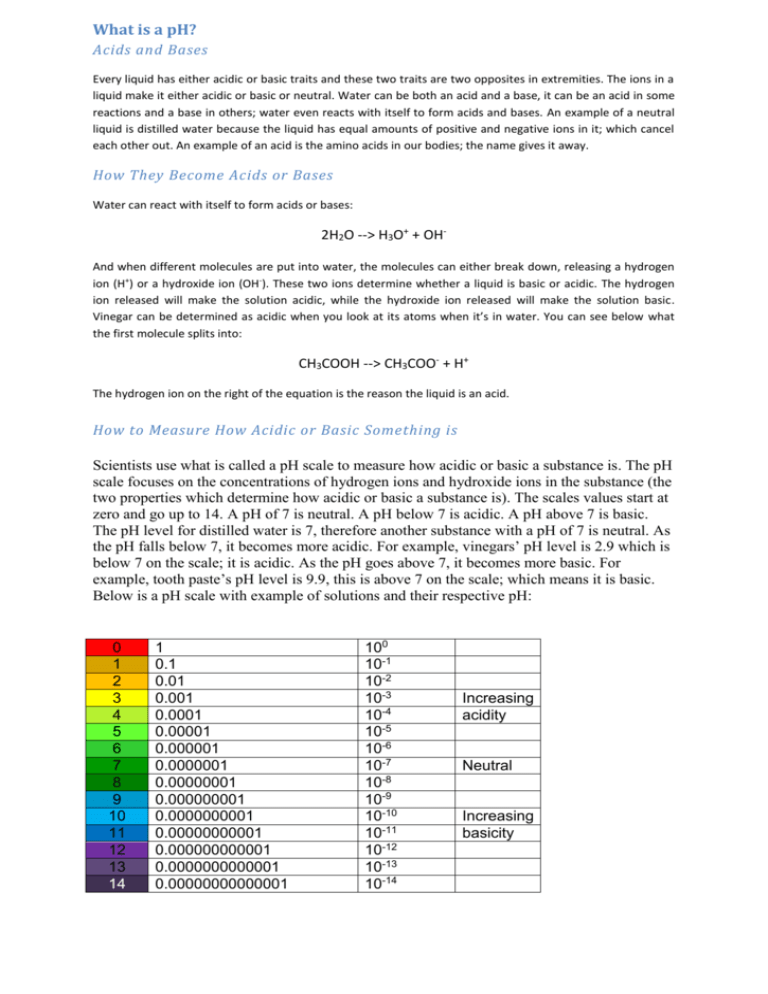
What is a pH? Acids and Bases Every liquid has either acidic or basic traits and these two traits are two opposites in extremities. The ions in a liquid make it either acidic or basic or neutral. Water can be both an acid and a base, it can be an acid in some reactions and a base in others; water even reacts with itself to form acids and bases. An example of a neutral liquid is distilled water because the liquid has equal amounts of positive and negative ions in it; which cancel each other out. An example of an acid is the amino acids in our bodies; the name gives it away. How They Become Acids or Bases Water can react with itself to form acids or bases: 2H2O --> H3O+ + OHAnd when different molecules are put into water, the molecules can either break down, releasing a hydrogen ion (H+) or a hydroxide ion (OH-). These two ions determine whether a liquid is basic or acidic. The hydrogen ion released will make the solution acidic, while the hydroxide ion released will make the solution basic. Vinegar can be determined as acidic when you look at its atoms when it’s in water. You can see below what the first molecule splits into: CH3COOH --> CH3COO- + H+ The hydrogen ion on the right of the equation is the reason the liquid is an acid. How to Measure How Acidic or Basic Something is Scientists use what is called a pH scale to measure how acidic or basic a substance is. The pH scale focuses on the concentrations of hydrogen ions and hydroxide ions in the substance (the two properties which determine how acidic or basic a substance is). The scales values start at zero and go up to 14. A pH of 7 is neutral. A pH below 7 is acidic. A pH above 7 is basic. The pH level for distilled water is 7, therefore another substance with a pH of 7 is neutral. As the pH falls below 7, it becomes more acidic. For example, vinegars’ pH level is 2.9 which is below 7 on the scale; it is acidic. As the pH goes above 7, it becomes more basic. For example, tooth paste’s pH level is 9.9, this is above 7 on the scale; which means it is basic. Below is a pH scale with example of solutions and their respective pH: 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 1 0.1 0.01 0.001 0.0001 0.00001 0.000001 0.0000001 0.00000001 0.000000001 0.0000000001 0.00000000001 0.000000000001 0.0000000000001 0.00000000000001 100 10-1 10-2 10-3 10-4 10-5 10-6 10-7 10-8 10-9 10-10 10-11 10-12 10-13 10-14 Increasing acidity Neutral Increasing basicity What is a Logarithmic Function? In the most basic terms of mathematics, a logarithmic function is known as an inverse exponential function for any number greater than zero. 𝑦 = 𝑙𝑜𝑔𝑏 𝑥 or 𝑥 = 𝑏 𝑦 , where x>0 It, in simple words, asks ‘which number is b raised to in order to get an answer of x?’. Logarithms are most commonly dealt with in base 10, for the ease of calculation, however Natural Logs, base e, are also extremely common too. Common Logs, where the base is any number, can be converted into either ‘Base 10’ or ‘Natural Logs’ using Laws of Logarithm. 𝑦 = 𝑙𝑜𝑔𝑏 𝑥 Y is the exponent/ power. B is the base number X is the number from the exponential equation. This graph is showing both a sketched logarithmic function, and an exponential function. Since they are inverses of each other, the graphs are essentially symmetrical. Take note that even though it seems the y-value for the logarithmic graph levels out, it doesn’t it can go on for an infinite number but will increase much slower than the exponential one. The x-value will also be a negative infinite value. (−∞, ∞). As it is not necessary to know how to work out the complex numbers by hand, when a logarithm is in a base different to base ten, you will need to change the base so that a calculator can handle it. The formula to do this allows you to choose any base, however base 10 is the most appropriate base to use 𝑙𝑜𝑔𝑐 𝑥 = 𝑙𝑜𝑔𝑚 𝑥 𝑙𝑜𝑔𝑚 𝑐 . A pH is a Logarithmic Function because? Consider the pH Scale: A pH of 14 has [H+] concentration of 10-14; that for a pH of 13 is 10-13 and the pattern follows. The formula used to calculate the [H+] concentration is: [H+] = 10-pH Thus: pH = -log [H+] It is important to note that the formula for the calculation of the pH has been derived from the formula for the calculation of [H+] ions. However, 10-pH is an exponential function and –log [H+] is a logarithmic function. They are both based on the same concept of indices however they are inverse of one another. The reason that pH is referred to as a logarithmic function because what determines the pH, [H+] concentration, is in fact an exponential function, which is the inverses of a logarithmic function. Since calculating pH requires us to calculate the negative log of the Hydrogen ion concentration, it becomes a logarithmic function. Graphing: Let’s graph pH Vs. [H+] Since: [H+] = 10-pH y = 10-x H+ Asymptote pH The function creates an asymptote with the x-axis. This is an important trait of a logarithmic function as it indicates that there is always some H+ ions present in a substance and therefore every substance can be compared with one another on the basis of acidity. An order of magnitude sea change, what’s in the fisherman’s basket? Consider the following: pH of 7 means that the Hydrogen ion concentration is 10-7 mol/L. pH of 6 means that the Hydrogen ion concentration is 10-6 mol/L. From an increase of acidity level by just 1 pH level, from 7 to 6, the pH level increased by 10 times as many ions: 10−6 10−7 = 10 This is called an exponential growth. This means that for every drop in pH, the level of acidity increases 10 times. This is the order of magnitude for the change in pH level in the sea. So what’s in the fisherman’s basket? Since the order of magnitude is 10, the contents of the fisherman’s basket are affected 10 times as much with just a single rise in the pH level. For instance, consider the case of Phytoplankton. They need carbonate ions (CO3-2) ,to react with calcium ions (Ca2+), found in the ocean, to build and maintain their exoskeleton. However, the concentration of these ions are decreasing due to an increase in the concentration of [H+] ions because they react together to produce bicarbonate ions: H+ + CO32- => HCO3If the concentration of H+ ions increases 10 times to what it was, then the concentration of carbonate ions drops to 1/10th of what it was. If the carbonate ions cannot be used to make Calcium Carbonate then this greatly affects the skeleton of the Phytoplankton. Healthy. Malformed Shell Plates. Corals, which are home to several thousand species of fish and other organisms, are greatly affected by ocean acidification as well. With an increase in Ocean Acidity The acidity level of the ocean is measured by something called a pH scale, which is a logarithmic scale. Ocean acidification is defined by a decrease in the pH of the ocean according to the pH scale. The logarithmic function comes from the base 10, because for every 1 unit increase on the pH scale the hydrogen ion [+H] levels increase by 10 times. This occurs due to a rise in the intake of CO₂ from the atmosphere. Ocean acidification will affect only specific parts of the ocean, as there is enough calcium carbonate to slowly neutralize the pH on a global scale. The decrease in species would be directly proportionate to the increase in CO₂ absorbed into the ocean. Explaining the processes related to the carbon cycle that take place in the ocean. Not the best explanation, however it shows the different depths, and what ions go where. Two possible reactions equate from the increase of CO₂ in the ocean. One would not be beneficial to the marine life; however it would not be too over whelming either. This reaction does create carbonate ions and results in a lower pH. CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3- ↔ 2H+ + CO32- At the current pH of the ocean a different reaction occurs, which consumes the carbonate ions and keeps the pH constant. CO2 + H2O + CO32- ↔ 2HCO3- Purely on estimation, a 0.1 unit decrease in pH is thought to be equivalent to a 26% increase in the hydrogen ions present. This is a large increase in the long run if it continues to build up. The first species affected will be the organisms which need the calcium and carbonate in the water, in order to construct their outer shells/ skeletons. These will include corals, phytoplankton, mussels, sea urchins, snails and other species. As the pH decreases so too does the availability of calcium carbonate for these organisms to use. This then means that the growth of coral reefs is slower and the end result is more prone to erosion. This occurs due to the liquids inside coral that photosynthesise to create the exo-skeleton needing a very specific pH to function therefore somewhat weakening the internal structure. Species that form the bottom of the food chain could also have a negative response to even the slightest change in the oceans pH. Since these species form the bottom of the food chain it could have a domino effect, where it’s like a building, the bottom falls and the rest follow. The pH would only have to be out by 1 unit (as it’s an increase of hydrogen ions to the ten) in order to effect the entire marine ecosystem. Some algae’s will have a positive effect out of an increase in CO₂ content found in the ocean, as it will assist in their photosynthesis. They will flourish, however the problem is that not all these algae’s are friendly, and some will wipe out the native species which were not as wide spread. Invertebrates and some fish could possibly end up with a build-up of calcium carbonate in their bodily fluids. This will cause; metabolic depression (lowered energy levels), lowered oxygen supply to the body, behavioural depression and low immune responses. All of these could affect the species breeding, therefore running the risk of extinction. Note however this is some fish not all, as scientists are still testing to see the effects on other marine life. At this current time it is only the smaller marine life that is known for definite will be affected by a change in pH. Therefore the larger marine life will be impacted due to a decrease in food source. Although an example is that of scientists being able to prove that a lobsters shell will thicken when exposed to lower pH level water. However in saying this, the lobsters suffer a decrease in energy which relates to less growth and fertility. Adaption is thought not possible in most circumstances, even amongst those species which breed relatively quickly. However this is in the hands of nature, so what scientists think could be incorrect, or something could happen between now and then. Ocean acidification however is pointed more at the surface water, not deep down as that is where the CO₂ is mostly absorbed. Remembering also that this is not a global scheme it is more certain parts most likely those closest to the civilised colonies of the world.