3) The Measure of All Things
advertisement

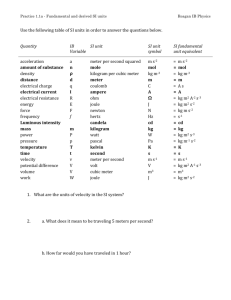
The Measure of All Things ABE425 Engineering Measurement Systems Dr. Tony Grift History of Le Système International d’unités 1791 March 30 —the new definition for the meter be equal to one tenmillionth of the length of the Earth's meridian along a quadrant through Paris, that is the distance from the equator to the north pole. Delambre Méchain Triangulation using the Borda repeating circle improved precision markedly by repeated observations Repeating circle surveying instrument During the “Terror” (1793-1794), the guillotine was used to execute more than 3,000 people per month Execution of Maximilien Robespierre, leader of the French Revolution, 1794 Dr. Joseph Guillotin The SI system was formalized in the Convention du Mètre, a treaty among 51 nations (2008) The International Bureau of Weights and Measures is an international standards organization, one of three such organizations established to maintain the International System of Units (SI) under the terms of the “Treaty of the meter” (Convention du Mètre). The organization is usually referred to by its French initials, BIPM (Bureau International des Poids et Mesures). The BIPM was created on 20 May 1875, following the signing of the meter Convention, a treaty among 51 nations (as of August 2008). It is based at the Pavillon de Breteuil in Sèvres, France, where it enjoys extraterritorial status. The seven base unit definitions 1. 2. 3. 4. 5. 6. 7. Length (distance) Mass Time (duration) Electric current Temperature Matter Luminous intensity (candela) Base units are defined, based on elementary physics relationships, but how can they be realized in a primary standard? How many direct electric units are there? Does this surprise you? Length definition: Meter 1790 — The French National Assembly decides that the length of the new meter would be equal to the length of a pendulum with a half-period of one second. 1791 — The new definition for the meter be equal to one ten-millionth of the length of the Earth's meridian along a quadrant through Paris, that is the distance from the equator to the north pole. 1795 — Provisional meter bar constructed of brass. 1799 — The French National Assembly specifies the platinum meter bar, as the final standard. 1889 — The International Committee for Weights and Measures Committee Generale des Poids et Mesures (CGPM) defines the meter as the distance between two lines on a standard bar of an alloy of platinum with ten percent iridium, measured at the melting point of ice. 1927 — The seventh CGPM adjusts the definition of the meter to be the distance, at 0 °C, between the axes of the two central lines marked on the prototype bar of platinum-iridium, this bar being subject to one standard atmosphere of pressure and supported on two cylinders of at least one centimeter diameter, symmetrically placed in the same horizontal plane at a distance prototype bar of platinum-iridium of 571 millimeters from each other. Length definition: Meter cont. 1960 — The eleventh International Committee for Weights and Measures defines the meter to be equal to 1,650,763.73 wavelengths in vacuum of the radiation corresponding to the transition between the 2p10 and 5d5 quantum levels (resonance) of the krypton-86 atom. 1983 — The seventeenth CGPM defines the meter as equal to the distance travelled by light in vacuum during a time interval of 1⁄299,792,458 of a second. 2002 — The International Committee for Weights and Measures (CIPM) recommends this definition be restricted to “lengths ℓ which are sufficiently short for the effects predicted by general relativity to be negligible with respect to the uncertainties of realization.” Mass definition: kilogram The kilogram was originally defined as the mass of one litre (or, equivalently, 1 dm3) of water (ice) at its melting point. This definition was later revised to specify a temperature of 4 °C. Mass is the only standard still based on a physical object. There are several proposed methods to link the kg to universal constants. Mass is the only standard that has a prefix (kilo) for historical reasons: Originally the kilogram was called the "grave", and the "gram" was an alternative name for a thousandth of a grave. After the French Revolution, the word "grave" carried negative connotations from the “Ancien Regime”, as a synonym for the title "count". The grave was renamed the kilogram. Mass definition : Platinum-iridium international prototype of the kilogram (IPK) kept in Sèvres (near Paris), France Since 1889, the SI system defines the magnitude of the kilogram to be equal to the mass of the International Prototype Kilogram or “IPK”. The IPK is made of a platinum alloy known as “Pt-10Ir”, which is 90% platinum and 10% iridium (by mass) and is machined into a right-circular cylinder (height = diameter) of 39.17 millimeters to minimize its surface area. Mass definition: Platinum-iridium international prototype of the kilogram (IPK) kept in Sèvres (near Paris), France The IPK and its six sister copies are stored at the International Bureau of Weights and Measures (BIPM) in an environmentally monitored safe in the lower vault located in the basement of the BIPM’s House of Breteuil in Sèvres on the outskirts of Paris. Three independently controlled keys are required to open the vault. 40 official copies of the IPK were made available to other nations (the USA got three) to serve as their national standards. These are compared to the IPK roughly every 50 years. The second had been based on a pendulum clock since 1658 to split a day in 24 hrs / 60 min / 60 sec In 1793, the Revolutionary Government in France decreed that the day should be Divided into 10 hours of 100 minutes, and the year into 10 months of 30 days. A pendulum clock built by Dutchman Christiaan Huygens, inventor of the pendulum clock (Horologium Oscillatorium), around 1658. Definition of Electric current (Ampere) Ampere (A): The ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross section, and placed 1 meter apart in vacuum, would produce between these conductors a force equal to 2*10-7 Newton per meter of length. The definition provides a link between electric current and force (Newton) which is related to kilogram, meter and second. This definition may be theoretically correct, but it is impossible to build an accurate realization. Ampere balance The ampere balance (also current balance or Kelvin balance) is an electromechanical apparatus used for the precise measurement of the SI unit of electric current, the ampere. The magnetic force between the two coils is measured by the amount of weight needed on the other arm of the balance to keep it in equilibrium. The accuracy of the current measurement is limited by the accuracy with which the coils can be measured, and their mechanical rigidity. Temperature in Kelvin kelvin (K): The kelvin, unit of thermodynamic temperature, is the fraction 1/273.16 of the thermodynamic temperature of the triple point of water. The International Temperature Scale of 1990 (ITS-90) is defined by two points: the absolute zero point (0 K or -273.15 °C) and the thermodynamic temperature of the water triple point, which is 273.16 K or 0.01 °C. There are many other reference points along the ITS-90 scale such as triple points, melting points and freezing points of metals Matter (in mole) In chemistry and physics, the Avogadro constant (symbols: L, NA) is the number of "elementary entities" (usually atoms or molecules) in one mole, that is (from the definition of the mole), the number of atoms in exactly 12 grams of carbon-12. The link with C-12 is artificial, and it links the Mole to the kg, which is a bad idea, since it is an artifact. NIST: “Traceability requires the establishment of an unbroken chain of comparisons to stated references each with a stated uncertainty “ The Système International is a beautiful consistent theoretical framework, but it is not in all cases conducive to realization. The culprits are the kg and the Ampere, but also the Kelvin and the Mole, neither of which is linked to a universal constant. Realization of the Length Unit meter The realization of the meter is conducted by means of lasers of a known and highly stable frequency. The primary standard consists of a helium-neon laser. The optical frequency of the laser is monitored by atomic clocks (realization of the second). The relative uncertainty of the meter realization with iodinestabilized HeNe lasers is 2.5*10-11, which corresponds to 1 mm in the circumference of the Earth. ‘Realization’ of the kg 40 copies of the original kg were made and they all vary in mass now. They gain mass through adsorption of atmospheric contamination onto their surfaces and lose mass due to cleaning. K48, came from the second batch of kilogram replicas to be produced. It was delivered to Denmark in 1949 with an official mass of 1 kg+81 µg. Still, its mass and that of the IPK diverged markedly in only 40 years; the mass of K48 was certified as 1 kg+112 µg during the 1988–1992 periodic verification. Realization of the second, using atomic clocks Time: The second is the duration of 9 192 631 770 periods of the radiation corresponding to the transition between two hyperfine levels of the ground state of the cesium 133 atom. In this instrument the frequency of a signal produced by a quartz oscillator is compared and adjusted to the resonance-frequency of a given transition in selected atoms. State of the art primary frequency standards exhibit a relative frequency uncertainty of 1e-15. Realization of the second, using atomic clocks cont. Atomic clock on a chip (NIST) NIST-F1 is a cesium fountain atomic clock that serves as the United States' primary time and frequency standard. It is so accurate that it will neither gain nor lose one second in more than 60 million years. Realization of an Electro Motive Force Volt standard (Weston Cell) The Weston cell, invented by Edward Weston in 1893, is a wetchemical cell (battery) that produces a highly stable voltage suitable as a laboratory standard for calibration of voltmeters. It was adopted as the International Standard in 1911. The Josephson junction is now used as the standard for the Electro Motive Force (Volt) In precision metrology, the Josephson effect provides an exactly reproducible conversion between frequency and voltage. Since the frequency is already defined precisely by the cesium standard, the Josephson effect is used, to give the definition of a volt (although, as of July 2007, this is not the official BIPM definition) Dr. Brian Josephson (Nobel prize, 1973, now ‘pioneer of the paranormal’) Vn n * f * h 2e n integer f frequency h Planck's constant e Elementary charge of an electron f * h Energy in the microwave radiation Josephson constant Kj 2e h One-volt NIST Josephson Junction array standard having 3020 junctions. This device operates at liquidhelium temperatures (4.2K). Microwave energy is fed through the fin-guide structure at the left. Josephson standard in a national calibration lab (METAS in Switzerland) The standard at METAS permits calibrations in the voltage range of -10 V to +10 V. At a level of 1.018 V, the measurement uncertainty of the system is ±5 nV. The Quantum Hall Effect is now used as the standard for resistance in Ohm Dr. Klaus Von Klitzing (Nobel prize, 1985) A voltage V drives a current I in the positive x direction. Normal Ohmic resistance is V / I. A magnetic field in the positive z direction shifts positive charge carriers in the negative y direction. This generates a Hall potential ( VH) and a Hall resistance (VH/ I ) in the y direction. (Kosmos, 1986) The Quantum Hall Effect produces quantized steps in resistance as a function of the magnetic field strength The resistance of this material in Ohm is quantized as a function of the magnetic field (Tesla) applied. The steps occur at resistance values that do not depend on the properties of the material but are given by a combination of fundamental physical constants multiplied by an integer. e2 where e is the elementary charge of an electron n h (1.602 176 487(40)×10−19 Coulomb and h is Planck's constant. n is integer. The resistance unit Rk, roughly equal to 25,812.8 ohms, is referred to as the Von Klitzing constant h Rk 2 =25812.807 e The Coulomb is now related to the Volt and Ohm standards and so is the Farad and Ampere The Josephson junction Volt standard, and Quantum Hall resistance standard (Von Klitzing constant) allow redefinition of the charge unit Coulomb which is equal to the charge of 6.241 509 629 152 65 * 1018 electrons 2e Hz =4.835979*1014 h Volt K * R 2e * h 2 e 2 j k 2 h e e Kj * Rk h 4 Rk 2 =2.5812807*10 e Kj C 1 6.241 509 629 152 65*1018 e The capacitance unit Farad can be derived from this unit as well: F C V Most importantly, the ampere (now linked to a force in N) can be redefined as the Coulomb per second: A C s Millikan’s experiment gave the charge of a single electron which can be validated against Hall & Josephson effects The experiment entailed balancing the downward gravitational force with the upward buoyant and electric forces on tiny charged droplets of oil suspended between two metal electrodes. By repeating the experiment for many droplets, he confirmed that the charges were all multiples of a fundamental value, and calculated it to be 1.5924(17)×10-19 C, within one percent of the currently accepted value of 1.602 176 487 (40)×10-19 C. They proposed that this was the charge of a single electron. 2 e Kj * Rk The future of the SI system How to modernize the SI system Define base units on realizations Meter based on speed of light Second based on atomic resonance frequency of cesium 133 Volt based on Josephson effect Electrical Resistance in Ohm based on Quantum Hall effect Amount of substance, based on Avogadro’s number Thermodynamic temperature based on Boltzmann’s constant Spectral luminous efficacy based on K 555 Proposed alternatives 1. Explicit constant definition: Fix all known constants and associate each unit with a constant 2. Explicit unit definition: Fix all known constants but do not associate each unit with a single constant 3. ‘Bake your own pizza”. Do as you please, link any unit with any physical constant, CGPM will provide ‘preferred methods’. Mills, I.M., Mohr, P.J., Quinn, T.J., Taylor, B.N., Williams, E.R. 2006. Redefinition of the kilogram, ampere, kelvin and mole: a proposed approach to implementing CIPM recommendation 1 (CI-2005). Metrologia 43: 227-246, doi:10.1088/0026-1394/43/3/006 Proposed alternative 1: Explicit constant definitions 1. Second: ground state hyperfine splitting transition frequency of cesium 133 atom 2. Meter: Speed of light in vacuum (this value has already been fixed) 3. Kg: Planck’s constant 4. Ampere: Elementary charge of an electron 5. Temperature: Boltzmann’s constant 6. Mole: Avogrado’s constant 7. Candela: spectral luminous efficacy of monochromatic radiation of frequency 133 Cs hfs 9 191 631 770 s 1 c0 299 792 458 ms -1 h = 6.626 0693(11) × 10-34 J s e = 1.602 176 53(14) × 10-19 C k = 1.380 6505(24) × 10-23 JK -1 N A = 6.022 1415(10) × 1023 mol-1 K 555 683 lumens Watt 1 “Such that’ construct: The International System of Units, the SI, is the system of units scaled such that the speed of light in vacuum c0 is exactly 299 792 458 metres per second, or such that the Planck constant is exactly….. kg derived?? kg Mass Speed of light c0 299 792 458 ms 1 meter kg=Ns2/m LENGTH If all base units would be defined based on known universal constants, the kg can become a derived unit. How do we make a realization though? Electrical and mechanical power are equal, can we couple them in some kind of electric motor device? Resonance Cesium atom CS 9 191 631 770 s 1 133 second Coulomb C=A*s Electric Charge Farad F=(C/V) Newton Capaci Force tance N=J/m TIME Kj 2e h Josephson junction KJ 2e Hz 4.835979*1014 h V Volt Ampere A=V/ ) Joule J=W*s=Nm Electric Energy Current (Work) VOLT RK h e2 Quantum Hall Effect h RK 2 25,812.807 e Ohm Watt Power W=V*A=J/s RESISTANCE Boltzmann constant k 1.3 806 505 JK 1 Kelvin THERMODYNAMIC TEMPERATURE Millikan charge of single electron experiment e 1.60217653*1019 Coulomb K J * RK e h 1 * 2 C 2 h e e Spectral luminance K 555 683 lumensW 1 Candela LUMINOUS INTENSITY Avogadro constant N A 6.0221415*1023 mol 1 mol Multiply by AMOUNT OF SUBSTANCE Divide by Redefine the kilogram using a Watt Balance In the NIST watt balance experiment, a kilogram test mass is placed on a balance pan that is connected to a coil of copper wire, which surrounds a superconducting electromagnet. If electric current is sent through the coil, then just as in an electric motor, electromagnetic forces are produced to balance the weight of the test mass. The apparatus measures this current and force. The apparatus also can move the coil vertically, and, like an electric generator, that induces a voltage. The velocity and voltage of the coil also are measured. These four measurements determine the relationship between mechanical and electrical power, which can be combined with other basic properties of nature to redefine the kilogram. F *v V * A The proposition of the Avogadro Project is to redefine the kilogram in terms of the Avogadro constant. By current definition, an Avogadro number of Carbon-12 atoms weigh exactly 12 grams. As such, the kilogram could be defined as the mass of 1000/12 * Avogadro's number of Carbon-12 atoms. The Avogadro constant itself is obtained from the ratio of the molar mass to the mass of an atom. For a crystalline structure such as silicon, the atomic volume is obtained from the lattice parameter and the number of atoms per unit cell. The atomic mass is then the product of the volume and density. The nominal diameter of a 1 kg Silicon sphere is 93.6 mm. In order to obtain an accuracy of 0.01 ppm in volume, the diameter must be known to a range of 0.6 nm, in other words, within one atom spacing. The roundness delta of the finished sphere is about 50 nm on a 93.6 mm diameter. It is believed to be the roundest object in the world. The English‘unit system’ What’s wrong with it, or what isn’t? Many units for the same thing. Volume: gallon, liquid quart, dry quart, liquid pint, dry pint, fluid ounce, teaspoon, tablespoon, minim, fluid dram, gill, peck, bushel. Strange conversions among many units. Johnny, there are 16 ounces in a pound, 12 inches in a foot, and 3 feet in a yard. Poor Johnny. You cannot remember all these conversion factors and why would you, it makes no sense anyway! If you cannot remember the conversions, how can you remember the formula? You don’t, you just look it up. Now what if your problem isn’t in the book? No realistic measure of small weights (lbs-oz- ??) You need dual sets of tools to work on your car People mix and match: we had 1/10 of an inch of rain. Our building uses 4,100 kgal of cooling water per year Many costly mistakes are linked to conversion issues, 125 million dollar Mars explorer, aircraft running out of fuel in midair, leaking seals in a nuclear power plant…. The awful invention of the ‘gauge’!!! Metric is for nerds! This causes a schism between the nerdy crowd and the “blue collar” crew. The persistent English unit system makes us look dumb and backward.. Things that make us look smart, innovative and modern The ENGLISH UNIT SYSTEM Forget Burma (Myanmar) and Liberia. The United States is the only developed country that is not officially metric! Lbs, oz, grain Inch, foot, yard, mile, rod Assignment Mass LENGTH Repeat the definition of units using the ENGLISH unit system. Electric Charge Capaci tance TIME Force Lbs, slug Electric Current VOLT Energy (Work) BTU HP Power RESISTANCE THERMODYNAMIC TEMPERATURE LUMINOUS INTENSITY Multiply by AMOUNT OF SUBSTANCE The English unit system has NO STANDARDS !!! Divide by The most important thing to remember The English unit system HAS TO GO!!! It is OUR responsibility and duty as (future) educators to cure this country from this illness. Let’s make Metric Day a departmental day of celebration 10/10/10 !!!! This will happen only once in your lifetime!