SI Units
advertisement

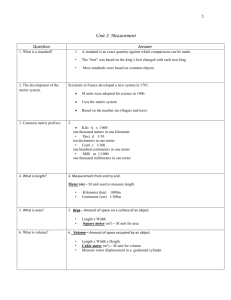
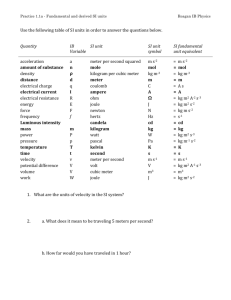
The SI System Measurements and Calculations Système Internationale d'Unités (SI) • an internationally agreed upon system of measurements. • Decimal system (all conversions based on multiples of 10) • SI consists of seven base units. Base Unit: defined unit in a system of measurement that is based on an object or event in the physical world, and is independent of other units. 1 mL = 1 cm3 1 milliliter is the same volume as 1 cubic centimeter. 1 mL of water has a mass of approximately 1g The mass of 1 milliliter of water is approximately 1 gram. 1 L of water has a mass of approximately 1 kg The mass of 1 liter of water is therefore approximately 1 kilogram. 1 m3 of water has a mass of approximately 1 t There are 1000 liters in a cubic meter, so the mass of 1 cubic meter of water is approximately 1000 kilograms or 1 metric ton. Mass of a nickel is 5 g A US nickel weighs 5 grams, and a penny weighs 2.5 grams. A typical doorknob is 1 m high Although there's no precise standard for doorknob heights, they're often about 1 meter above the floor. The diameter of a CD or DVD is 12 cm A CD or DVD is 12 centimeters (120 millimeters) across. The diameter of the center hole is 15 millimeters. 1 ha is 1002 m2 1 hectare is 10 000 square meters, equivalent to the area of a square 100 meters on a side. A football field is about 100 meters long, so imagine a square the length of a football field on each side, and that's 1 hectare. Base Units The SI base unit of time is the second (s), based on the frequency of radiation given off by a cesium-133 atom. The SI base unit for length is the meter (m), the distance light travels in a vacuum in 1/299,792,458th of a second. The SI base unit of mass is the kilogram (kg), about 2.2 pounds SI Base Units MANY DERIVED UNITS Quantity Unit Abbreviation Speed = Length/Time meter/second m/s Volume =(Length) 3 (meter) 3 m3 Density = Mass/Volume kg/m 3 kg/m 3 Acceleration = speed/time m/s 2 m/s 2 Force = Mass x Acc kg m/ s 2 N (Newton) Pressure = Force/Area kg/m s 2 Pa (Pascal) Energy = Force x Length kg m 2 / s 2 J (Joule) Prefix Symbol Exponential yotta zetta exa peta tera giga mega kilo hecto deca Y Z E P T G M k h da 1024 1021 1018 1015 1012 109 106 103 102 101 no prefix (base unit) deci centi milli micro nano pico femto atto zepto yocto d c m m n p f a z y 100 10¯1 10¯2 10¯3 10¯6 10¯9 10¯12 10¯15 10¯18 10¯21 10¯24 Converting between SI Units Use the chart like this: 1 prefixed unit = exponential value of the prefix 1 cm = 10-2 m 1 nm = 10-9 m 1 Mm = 106 m Example: Measurements of Length Base Unit: the Meter Centimeter Millimeter Nanometer Micrometer Picometer Measurement of Mass Base Unit: the kilogram (the gram is too small) only prefixed base unit Measurement of Time Base Unit: the second Measurement of Temperature A measure of how hot or how cold something is A quantity that determines the direction of heat flow: warmer to cooler Three temperature scales commonly used: Celsius Kelvin (absolute) Fahrenheit To Convert F to C and C to F 1.8 degrees F = 1 degree C F = 32 + (1.8 x degrees C) Example: Convert 22 degrees C to degrees F C= F – 32/1.8 Example: Convert 98.6 degrees F to degrees C. To Convert Celsius to Kelvin 0 degrees C = 273 K K = C + 273 C = K -273 Examples: Convert 100 degrees C to Kelvin. Convert 475 K to degrees C Convert 250 K to degrees C. Derived Units Volume and Density Calculations Volume Base Unit : m 3 Not practical because it is very large Commonly used: dm 3 Also called “THE LITER (L)” 1 dm = 10 cm 1 cm 3 = 1mL 1 dm 3 = 1 L NOTE: 1 dm 3 = (10 cm) 3 = 1000 mL= 1000 cm 3 = 1L Example: Calculate the volume of a cube that is 3.30 cm on each side. Density Mass to volume ratio The mass of a unit volume density = mass/volume d = m/v Examples Calculate the density of a piece of glass with a mass of 6.65 g and a volume of 2.95 mL. Calculate the thickness of an aluminum foil 15.38 cm long and 14.39 cm wide. The mass of the foil is 1.4939 g. The density of aluminum is 2.70 g/cm 3 . (HINT: We consider the Aluminum foil to be a rectangular solid). Dimensional Analysis (Factor Label) Method of calculating using the units Makes word problems and chemistry calculations easy! Any unit can be converted to another by using appropriate conversion factors Appropriate conversion factors Come from equality statements 1 kg = 103 g 1 hr = 60 minutes 1 mm = 10-3 m Conversion factors (equalities written as fractions) Which factor do I use? Start unit x final unit = final unit start unit Example On a picnic, 162 students are each given 2 hot dogs. If there are 9 hot dogs per pound, priced at $ 4 per 3 pounds, what is the cost of the hot dogs? Note that the following conversion factors can be obtained from the text of the problem: 1 student = 2 hotdogs 9 hot dogs = 1 lb $4 = 3 lbs To begin solving the problem, start with a known and keep your goal in mind: Note that all the units (except $) cancel out!