Chapter 17
advertisement
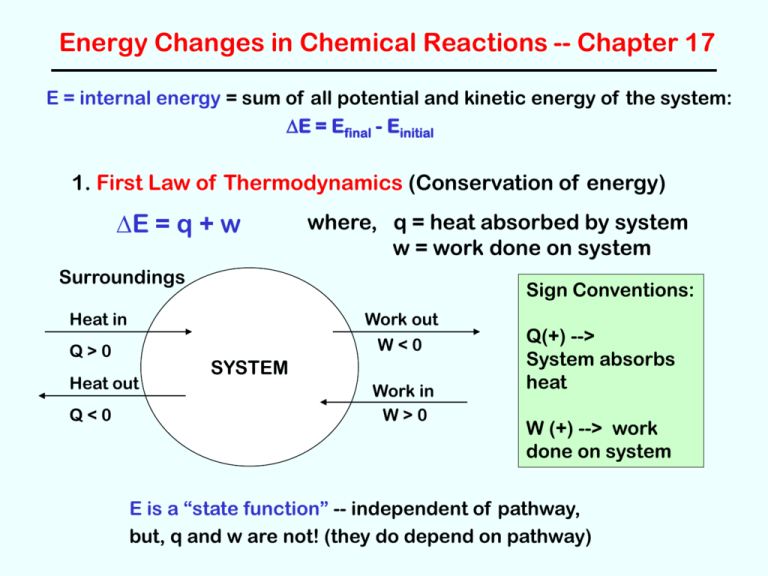
Energy Changes in Chemical Reactions -- Chapter 17
E = internal energy = sum of all potential and kinetic energy of the system:
DE = Efinal - Einitial
1. First Law of Thermodynamics (Conservation of energy)
DE = q + w
where, q = heat absorbed by system
w = work done on system
Surroundings
Sign Conventions:
Heat in
Work out
W<0
Q>0
Heat out
Q<0
SYSTEM
Work in
W>0
Q(+) -->
System absorbs
heat
W (+) --> work
done on system
E is a “state function” -- independent of pathway,
but, q and w are not! (they do depend on pathway)
Entropy and Spontaneity
(usually small)
Two factors affect any change or reaction
(a) Enthalpy (H) (Recall that DH = DE + PDV)
exothermic processes (negative DH) tend to be
spontaneous, but not always
(b) Entropy (S) – degree of disorder or randomness
Change in entropy:
DS = Sfinal - Sinitial
For a reaction:
DS = Sproducts - Sreactants
Positive DS means:
an increase in disorder as reaction proceeds
products more disordered (random) than reactants
For a reaction, positive DS favors spontaneity
Entropy and Second Law
Entropy (S) increases with the number of energetically equivalent
ways to arrange the components of a system to achieve a particular
state.
Trends:
•
•
•
•
In general: Sgas >> Sliquid > Ssolid
larger mass => more entropy
more complex structure => more entropy
more atoms in molecule => more entropy
Second Law of Thermodynamics
“…for any spontaneous process, the overall entropy of the
universe increases…”
A spontaneous process can have a negative DS for the system only if
the surroundings have a larger positive DS.
DSuniv = DSsys + DSsurr
where DSsurr =
–DHsys
T
Third Law
Third Law of Thermodynamics
“…the entropy of a pure crystalline substance equals zero at
absolute zero…”
S = 0 at T = 0 K
(a) Standard Entropy (at 25 ºC) = Sº
[Table 17.2, p789]
(note the units!)
Entropy change for a reaction:
(e.g. J/mole K)
DSº = S Sº(products) - S Sº(reactants)
Gibbs Free Energy
(b) Gibbs Free Energy = G
Defined as:
G = H - TS
– A combination of enthalpy and entropy effects
– Related to maximum useful work that system can do
For a process (e.g. a reaction),
DG = DH - TDS
For any spontaneous change, DG is negative!!!
(i.e. the free energy must decrease)
Standard Free Energy
DGº (at 1 atm)
generally used to decide if a reaction is
spontaneous (Yes, if negative)
Three ways to obtain DGº for a reaction
(a) from DHº and DSº
requires DHºf and Sº data for all reactants and
products
DGº = DHº - TDSº
DHº = SDHºf(products) - SDHºf(reactants)
DSº = SSº(products) - SSº(reactants)
Determining DGº
Three ways to obtain DGº for a reaction….
(b) from standard “Free Energies of Formation” DGºf
DGº = SDGºf(products) - SDGºf(reactants)
where DGºf is the free energy change for the formation of
one mole of the compound from its elements, e.g.
DGºf for Al2(SO3)3(s) equals DGº for the following reaction
2 Al(s) + 3 S(s) + 9/2 O2(g) --> Al2(SO3)3(s)
Manipulating Chemical Equations to Get DGº
If reaction is reversed, change sign of DG°.
If reaction is multiplied or divided by a factor, apply same
factor to DG°.
DG° for overall reaction = sum of DG° values for individual
reactions.
Problem
Given the following thermochemical reactions:
(eq 1) C2H4(g) + 3 O2(g) --> 2 CO2(g) + 2 H2O(l) DG° = -1098.0 kJ/mol
(eq 2) C2H5OH(l) + 3 O2(g)--> 2 CO2(g)+ 3 H2O(l) DG° = -975.2kJ/mol
Calculate DG° for the following reaction:
C2H4(g) + H2O(l) --> C2H5OH(l)
Example Problem, cont.
Reverse 2nd reaction to put C2H5OH on product side then
rewrite 1st equation and add them together.
(eq 2)
2 CO2(g) + 3 H2O(l) --> C2H5OH(l) + 3 O2(g)
DH° = + 975.2 kJ/mol (note the sign change!!!)
(eq 1)
C2H4(g) + 3 O2(g) --> 2 CO2(g) + 2 H2O(l)
DH° = -1098.0 kJ/mol
Net:
C2H4(g) + H2O(l) --> C2H5OH(l)
{note: 3 O2, 2 CO2, and 2 H2O cancel out}
DH° = DH°1 + DH°2 = 975.2 + (-1098.0) = -122.8 kJ/mol
Effect of Temperature on DG
(a) Effect of Temperature on DG
DG depends on DH and DS:
DG = DH – TDS
but DH and DS are relatively independent of temperature, so
DG at some temperature T can be estimated.
DGºT
≈
DHº298 - TDSº298
Free Energy and Equilibrium
(b) for a system at equilibrium:
Gproducts = Greactants
and
DG = 0
since DG = DH - TDS = 0
DH = T DS
or
T = DH/DS
Example Problem
Given the following, determine the normal boiling point of
mercury (Hg).
DHvaporization of Hg = 60.7 kJ/mole
entropies: liquid Hg:
gaseous Hg:
Equilibrium:
Hgliq
Sº = 76.1 J/mole K
Sº = 175.0 J/mole K
Hggas
DG = 0
T = DH/DS
so, DH - TDS = 0
or
T = [60.7 x 103 J/mole]/[(175.0 - 76.1) J/mol K]
= 614 K = 341 ºC
Free Energy and Equilibrium Constant
(c) Relationship between DGº and Equilibrium Constant (K)
For any chemical system:
DG = DGº + (RT) ln Q
If DG is not zero, then the system is not at equilibrium. It will
spontaneously shift toward the equilibrium state.
At Equilibrium:
∴
DG = 0
and
Q=K
DGº = -RT ln K
for gaseous reactions: K = Kp
for solution reactions: K = Kc
{units of DG must match those of R value}
Free Energy and Equilibrium (cont.)
DGº = – RT ln K
K values can be determined from thermodynamic data !
when K > 1
DGº is negative
∴ spontaneous reactions have
large K and negative DGº values
Sample Problem
1. Consider the gas-phase reaction: N2O5(g) + H2O(g) --> 2
HNO3(g) and the following thermodynamic data.
Compound
DHºf (kJ/mole)
Sº (J/mole K)
N2O5(g)
11.0
356
H2O(g)
- 242
189
HNO3(g)
- 174
156
(a) Decide whether or not the above reaction is spontaneous at
25 ºC by calculating the value of the appropriate
thermodynamic quantity.
(b) Calculate the temperature (in ºC) at which the above
reaction should have an equilibrium constant (Kp) equal to
1.00.
Sample Problem
1. Consider the gas-phase reaction: N2O5(g) + H2O(g) --> 2
HNO3(g) and the following thermodynamic data.
Compound
DHºf (kJ/mole)
Sº (J/mole K)
N2O5(g)
11.0
356
H2O(g)
- 242
189
HNO3(g)
- 174
156
(a) Decide whether or not the above reaction is spontaneous at
25 ºC by calculating the value of the appropriate
thermodynamic quantity.
(b) Calculate the temperature (in ºC) at which the above
reaction should have an equilibrium constant (Kp) equal to
1.00.
(a) DG = – 48 kJ/mol, which is negative, so spontaneous.
(b) 229 ºC
Sample Problem
For a certain reaction, DH° = -95.2 kJ and DS° = -157 J/K.
Determine DSsurr and DSuniv (in J/K) for this reaction at 850 K.
Based on these results, is the reaction spontaneous at this
temperature?
Sample Problem
For a certain reaction, DH° = -95.2 kJ and DS° = -157 J/K.
Determine DSsurr and DSuniv (in J/K) for this reaction at 850 K.
Based on these results, is the reaction spontaneous at this
temperature?
• Answer: DSsurr = +112 J/K
• DSuniv = -45 J/K
• Not spontaneous, because DSuniv would decrease, which is
against the 2nd Law of Thermodynamics.









