Thermodynamics Problem Set Solutions: Chemistry I-H
advertisement
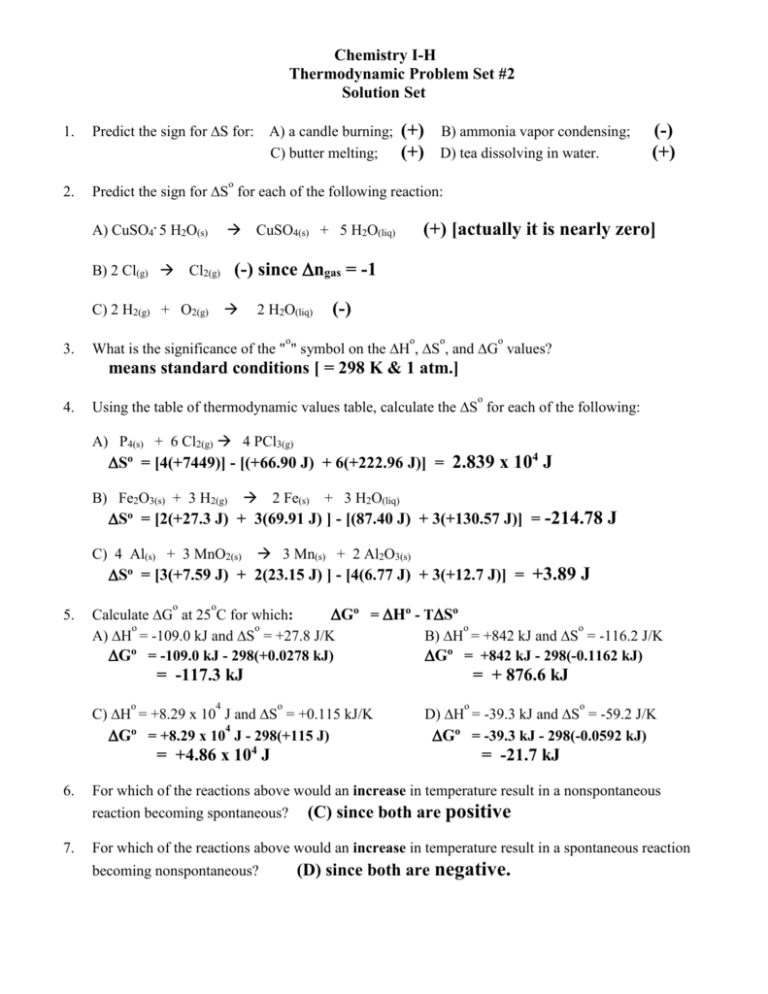
Chemistry I-H Thermodynamic Problem Set #2 Solution Set 1. Predict the sign for S for: 2. Predict the sign for S for each of the following reaction: (+) (+) B) ammonia vapor condensing; D) tea dissolving in water. (-) (+) o A) CuSO4. 5 H2O(s) B) 2 Cl(g) Cl2(g) (+) [actually it is nearly zero] CuSO4(s) + 5 H2O(liq) (-) since ngas = -1 C) 2 H2(g) + O2(g) 3. A) a candle burning; C) butter melting; 2 H2O(liq) (-) o o o o What is the significance of the " " symbol on the H , S , and G values? means standard conditions [ = 298 K & 1 atm.] 4. o Using the table of thermodynamic values table, calculate the S for each of the following: A) P4(s) + 6 Cl2(g) 4 PCl3(g) So = [4(+7449)] - [(+66.90 J) + 6(+222.96 J)] = 2.839 x 104 J B) Fe2O3(s) + 3 H2(g) 2 Fe(s) + 3 H2O(liq) S = [2(+27.3 J) + 3(69.91 J) ] - [(87.40 J) + 3(+130.57 J)] = -214.78 J o C) 4 Al(s) + 3 MnO2(s) 3 Mn(s) + 2 Al2O3(s) S = [3(+7.59 J) + 2(23.15 J) ] - [4(6.77 J) + 3(+12.7 J)] = +3.89 J o 5. o o Calculate G at 25 C for which: Go = Ho - TSo o o o o A) H = -109.0 kJ and S = +27.8 J/K B) H = +842 kJ and S = -116.2 J/K Go = -109.0 kJ - 298(+0.0278 kJ) Go = +842 kJ - 298(-0.1162 kJ) = -117.3 kJ o 4 = + 876.6 kJ o C) H = +8.29 x 10 J and S = +0.115 kJ/K 4 Go = +8.29 x 10 J - 298(+115 J) = +4.86 x 104 J o o D) H = -39.3 kJ and S = -59.2 J/K Go = -39.3 kJ - 298(-0.0592 kJ) = -21.7 kJ 6. For which of the reactions above would an increase in temperature result in a nonspontaneous reaction becoming spontaneous? (C) since both are positive 7. For which of the reactions above would an increase in temperature result in a spontaneous reaction becoming nonspontaneous? (D) since both are negative. -28. Calculate the following for the given reaction: o A) H 2 NO(g) + O2(g) 2 NO2(g) o o B) S = [2(240.45 j)] -[2(210.62) +(205.0)] = 2(33.84 kJ) - 2(90.37 kJ) = -145.34 joules C) the temperature at which G = 0 T = H / S = -113.06 kJ / -0.14534 kJ = -113.06 kJ 9. = 778 K o The normal boiling point of trichloromethane (CHCl3) is 61.0 C; its molar heat of . vaporization (Hvap) is 31.3 kJ/mole. The standard entropy of this liquid is 202.9 J/mole K. o o Determine G at 61.0 C. Go = Ho - TSo = -31.3 kJ - (334 K)(0.2029 kJ) = -99.1 kJ 10. What is the normal freezing point for a substance if its heat of fusion is 34.5 kJ/mole and its entropy . in the solid phase is +56.7 J/mole K? T = H / S = 34.5 kJ / 0.0567 kJ = 608 K 11. A Which of the following quantities can be taken to be fairly independent of pressure? A) H for a reaction; B) S for a reaction; C) S for a substance; D) G for a reaction. 12. Criticize each of the following statements: o A) When the value for G is positive, the reaction cannot occur. The reaction can occur, but it needs an outside source of energy to sustain it. This “outside source” is known as the activation energy. o B) When the values for Ho and S are both positive, the reaction is nonspontaneous at all temperatures. G = H - TS At a high enough temperature, the reaction may be spontaneous because the TS factor may offset the H factor.