Document
advertisement

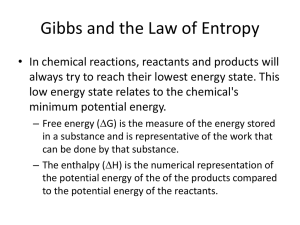
Period ________ Name ______________________________ ENTROPY Entropy is a measure of the degree of disorder. (S or S if talking about change) The universe tends to move toward higher entropy (or in other words, reactions that increase entropy are favored.) Solids have the lowest entropy, gases the highest. Liquids and aqueous ions are in between. High S Low S Gas Liquid/Aqueous Solid When chemical reactions move toward higher disorder, we say it is a positive S. If it moves toward more order, we say it is a negative S. If the type of phase does not make it clear how entropy is changing, you can also look at how many moles of each phase are present on each side of the reaction. For example, a reaction that goes from 1 mole of gas to 3 moles of gas would be an increase in entropy because there is more gas now (and more disorder). Determine S for the following reactions: (circle the appropriate answer) 1. 2KClO3(s) 2KCl(s) + 3O2(g) Increase Decrease 2. H2O(l) H2O(s) Increase Decrease 3. N2(g) + 3H2(g) 2NH3(g) Increase Decrease 4. NaCl(s) Na+(aq) + Cl-(aq) Increase Decrease 5. KCl(s) KCl(l) Increase Decrease 6. CO2(s) CO2(g) Increase Decrease 7. H+(aq) + CH3COO-(aq) HCH3COO (l) Increase Decrease 8. C(s) + O2(g) CO2(g) Increase Decrease 9. H2(g) + Cl2(g) 2HCl(g) Increase Decrease 10. Ag+(aq) + Cl-(aq) AgCl(s) Increase Decrease 11. 2N2O5(g) 4NO2(g) + O2(g) Increase Decrease 12. 2Al(s) + 3I2(s) 2AlI3(s) Increase Decrease 13. H+(aq) + OH-(aq) H2O(l) Increase Decrease 14. 2NO(g) N2(g) + O2(g) Increase Decrease 15. H2O(g) H2O(l) Increase Decrease Period ________ Name ______________________________ GIBBS FREE ENERGY G = H – (TS) T is temperature in Kelvins, H is change in enthalpy or heat of reaction, S is change in entropy G can be used to predict the spontaneity of a reaction: G > 0 (+) means reaction is not spontaneous G < 0 (-) means reaction is spontaneous Note: Units for H are usually kJ and units for S are usually J, so you must fix that before cranking out any G values! Complete the table below for G. You options are: always spontaneous, never spontaneous, spontaneous at high temperatures, spontaneous at low temperatures. H S G + + + + Answer the questions below: 1. The conditions under which G is always negative are when H is _____________ and S is ___________. 2. The conditions under which G is always positive are when H is _____________ and S is ___________. 3. a) When you have an endothermic reaction that is going towards more disorder, high or low (circle one) temperature favors spontaneity. b) When you have an exothermic reaction that is going towards less disorder, high or low (circle one) temperature favors spontaneity. Answer problems 4-6 with: always, sometimes, or never. Note: If energy is on the reactants side, the reaction is endothermic. If energy is on the products side, the reaction is exothermic. 4. The reaction: NaOH(s) Na+(aq) + OH-(aq) + energy will ____________ be spontaneous. 5. The reaction: energy + 2H2(g) + O2(g) 2H2O(l) will ____________ be spontaneous. 6. The reaction: energy + H2O(s) H2O(l) will _____________ be spontaneous. 7. What is the value of G if H = -32.0kJ, S=+25.0kJ/mol•K, and T=293K? _______________kJ 8. Is the reaction in problem 7 spontaneous? (circle one) yes no 9. What is the value of G if H = +12.0kJ, S=-5.00kJ/mol•K, and T=290.K? _______________kJ 10. Is the reaction in problem 9 spontaneous? (circle one) yes no