Essential Idea
Compounds of carbon, hydrogen and oxygen are
used to supply and store energy.
Understandings.
• Monosaccharide monomers are linked
together by condensation reactions to form
disaccharides and polysaccharide polymers.
IB Assessment Statement
• List three examples each of
monosaccharides, disaccharides and
polysaccharides. The examples used
should be: glucose, galactose and fructose;
maltose, lactose and sucrose; starch,
glycogen and cellulose.
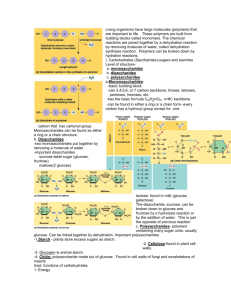
Sugars: monosaccharides
• Monosaccharides have molecular formulas that
are usually multiples of CH2O
• Monosaccharide is a molecule made up of one
type of sugar
• Glucose is the most common monosaccharide
Monosaccharide: Glucose
Copyright Pearson Prentice Hall
Monosaccharide: Ribose
Copyright Pearson Prentice Hall
Disaccharides: Two Sugars
• A disaccharide is formed when a dehydration
reaction joins two monosaccharides
Animation: Disaccharides
Disaccharides: Two Sugars
• Disaccharide
examples:
– Lactose formed
from a bond
between
Galactose and
glucose
Animation: Disaccharides
Disaccharides: Two Sugars
• Disaccharide
examples:
– Sucrose formed
from a bond
between
fructose and
glucose
Animation: Disaccharides
Application
• Application: Structure and function of cellulose
and starch in plants and glycogen in humans.
Skill:
• Use of molecular visualization software to
compare cellulose, starch and glycogen.
Polysaccharides: Many Sugars
• Starch
– Formed from
many glucose
molecules
– Used for energy
storage in plants
Animation: Disaccharides
Polysaccharides: Many Sugars
• Glycogen
– Formed from
many glucose
molecules
– Energy storage
for animals
Animation: Disaccharides
Polysaccharides: Many Sugars
• Cellulose
– Formed from
many glucose
molecules
– Structural
support for
plants
Animation: Disaccharides
Polysaccharide Comparisons
Know the structures of the following carbohydrate
• Monosaccharides
• glucose, galactose,
fructose
•Disaccharides
•maltose, lactose and
sucrose
•Polysaccharides
•Starch, glycogen,
cellulose
IB Assessment Statement
• State one function of glucose, lactose and
glycogen in animals, and of fructose, sucrose and
cellulose in plants.
Carbohydrate functions in Animals
• Glucose – used as a substrate for
respiration or converted to glycogen for
storage.
• Lactose – produced by mammary glands and
secreted in milk as an important part of the
diet of young mammals
• Glycogen – energy storage
Carbohydrate functions in Plants
• Glucose – a product of photosynthesis.
• Fructose – produced as an intermediate
substrate during glucose breakdown during
respiration. Used in production of sucrose
• Cellulose – component of cell walls/ structural
support in plants
• Starch – energy storage
Carbohydrate tutorial
Click on the Carbohyrdate tutorial below
•http://www.wisconline.com/objects/ViewObject.aspx?ID=AP1310
4
IB Assessment Statement
• Outline the role of condensation & hydrolysis
reaction the relationships between
monosaccharides, disaccharides and
polysaccharides;
Hydrolysis vs. Condensation
Hydrolysis
Condensation
•Adds water
•Removes water
•Breaks down polymers into
monomers
•Forms new bonds between
monomers forming polymers
•Example: Breaks down
starch into glucose
•Example: glucose and fructose
are bonded together to form
sucrose
Building and Breakdown disaccharides and
Polysaccharides
• Condensation (dehydration)
and Hydrolysis Reactions
One Type of Condensation (dehydration) Reactions
1.
Occurs between monosaccharide and forms
disaccharides and polysaccharides
Disaccharides & Dehydration/ Condensation
Reaction
• When two monosaccharides join together a hydrogen is
released from one monosaccharide & a hydroxide is
removed from another
• Hydrogen and hydroxide bond together to form water
Animation: Disaccharides
Condensation (dehydration) Reaction
in carbohydrates
glucose
glucose
glucose
Fructose
Starch
Sucrose
One type of hydrolysis reactions
1.
Breakdown of disaccharide or polysaccharides into
monosaccharide
Flashcards
Make a flashcard of the following:
1st side but the name:
•
•
•
•
•
•
•
•
glucose,
Lactose
Glycogen (describe structure)
fructose,
sucrose
Cellulose (describe structure)
Ribose
Starch (describe structure)
2nd side put
• State one function of glucose, lactose, ribose and glycogen
in animals,
• and of fructose, sucrose, ribose and cellulose in plants.
• Draw/ describe the structure of the molecule
Understandings.
• Triglycerides are formed by condensation from
three fatty acids and one glycerol.
Lipids are a diverse group of hydrophobic
molecules
• Lipids occur in living things
as animal fat and plant oils
• The unifying feature of lipids
is having little or no
attraction for water
• Lipids are hydrophobic
because they consist mostly
of hydrocarbons, which
form nonpolar covalent
bonds
• The most biologically
important lipids are fats,
phospholipids, and
steroids
Fats and Oils
Fats are constructed from two types of smaller
molecules: glycerol (an alcohol) and fatty acids
• Glycerol is a three-carbon alcohol with a
hydroxyl group (--OH) attached to each carbon
• A fatty acid consists of a carboxyl group
(--COOH) attached to a long carbon skeleton
Structure of fat and oils
Fatty acid
Glycerol
•
Fats and oils are compounds called triglycerides
• In a fat, three fatty acids are joined to glycerol by
an ester linkage a triglyceride
• In cells, enzymes catalyze the formation of
triglyerides, and also the breakdown of glycerides
by hydrolysis
Below is a figure representing the Structure of Triglycerides
Ester linkage
Fat molecule (triacylglycerol)
IB Assessment Statements
• Outline the role of condensation and hydrolysis in
the relationships between between fatty acids,
glycerol and triglycerides; and between amino
acids and polypeptides.
Condensation reaction between glycerol &
fatty acids form lipids
Fatty acid
Glycerol
Phospholipids
• Phospholipids has a similar chemical structure to
triglycerides.
• In a phospholipid, one of the fatty acids is replaced by a
phosphate group (--PO4)
• The over structure of a phospholipid consists of
– two fatty acids
– and a phosphate group
–
attached to glycerol
• The two fatty acid tails are hydrophobic, but the
phosphate group and its attachments form a hydrophilic
head
LE 5-13
Choline
Phosphate
Glycerol
Fatty acids
Hydrophilic
head
Hydrophobic
tails
Structural formula
Space-filling model
Phospholipid symbol
• When phospholipids are
added to water, they selfassemble into a bilayer,
with the hydrophobic
tails pointing toward the
interior
• The structure of
phospholipids results in a
bilayer arrangement
found in cell
membranes
• Phospholipids are the
major component of all
Cell Membrane
Hydrophilic
head
Hydrophobic
tails
WATER
WATER
Lipid Tutorial Below:
Click below for the lipid tutorial
• http://www.wisconline.com/objects/ViewObject.aspx?ID=AP13
204
Understandings.
• Fatty acids can be saturated, monounsaturated
or polyunsaturated.
• Unsaturated fatty acids can be cis or trans
isomers.
Mono vs Poly UNSATURATED FATs
• Monounsaturated
Fats– One double bond in
the hydrocarbon chain
• Polyunsaturated Fats
– More than one double
bond exists in the
hydrocarbon chain
Saturated vs Unsaturated
fatty acids
• Polyunsaturated fats are
hydrogenated or partially
hydrogenated,
• Hydrogenated means
that double bonds in the
fatty acid are broken and
hydrogen are added.
• Poly and Mono
Unsaturated fats
becomes saturated
through the process of
hydrogenation.
Saturated (trans) vs. Unsaturated (cis)
• Mono and Poly Unsaturated fatty acids are
naturally curved.
• Saturated fatty acids are straight.
Trans vs. Cis
• Cis fatty acids are unsaturated, contain a
double bond in the fatty acid chain and are
curved.
– An example of a cis fatty acid is Omega -3
• Trans fatty acid are saturated, no double
bonds and straight.
– Vast majority of trans fatty acid are the result
of food process (i.e. hydrogenation)
CHD is coronary heart
disease
Why are Trans fats bad? VIDEOS
• Simple video
– https://www.youtube.com/watch?v=mYM7B2RfpdE
• GOOD VIDEO BELOW:
– https://www.youtube.com/watch?v=7kLLI2GluDE
• Lipid Video
– https://www.youtube.com/watch?v=ulIjtl4FPDQ
• Biochemistry & human physiology of fat in the
blood ( 1 hour long lecture)
– https://www.youtube.com/watch?v=_oLXa4SfsVs
Omega-3 and Omega 6 Fatty Acids
The name
omega 3
and omega
6 comes
from which
numbered
carbon has
the double
bond.
Nature of Science
• Evaluating claims—health claims made about
lipids in diets need to be assessed. (5.2)
Why are Trans fats bad?
• The Shape of trans fats make them bad for your
cardiovascular system.
–
Saturated trans fats are linear and thus they lay flat
against your arteries making is more difficult for them to
flow with your passing blood.
–
These linear, saturated, trans fatty acids combine with
cholesterol and form a substance called plaque and can be
deposited along the walls of your arteries blocking or
slowing blood flow. It this happens in the coronary arteries
you can have a heart attack.
Application
• Application: Lipids are more suitable for longterm energy storage in humans than
carbohydrates.
Energy Content in Food
• Fats contain more than twice as much energy
per 100 grams than carbohydrates and
proteins
• Carbohydrates: 1,760 kJ per 100 g
• Proteins: 1,720 kJ per 100 g
• Fats: 4,000 kJ per 100 g
Skills
• Determination of body mass index by
calculation or use of a nomogram.
• A.2.5 Calculate body mass index (BMI) from
the body mass and height of a person
• A.2.6 Distinguish, using the body mass
index, between being underweight, normal
weight, overweight and obese
• • Underweight – below
18.5
weight – 18.5 to 24.9
• • Overweight – 25 to
29.9
above 30.0
• Normal
• Obese –
Body Mass Index