File
advertisement

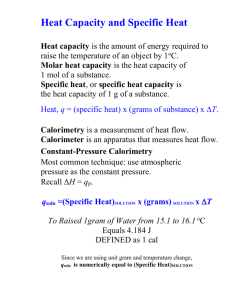
Unit 1: Chemistry Basics 1.52 Calorimetry Textbook ch 5.5 © 2009, Prentice-Hall, Inc. Big Idea 5: The laws of thermodynamics describe the essential role of energy and explain and predict the direction of changes in matter. Students will be able to demonstrate understanding by laboratory investigation, analysis of data and creation of models. Learning Objectives : SWBAT: •Calculate the heat transferred in a process from temperature measurements together with specific heat capacities Calorimetry is Another Way to Measure H values We measure H through calorimetry, which measures heat flow Two types of calorimetric or energy equations 1) Within a Phase – • These will have a change in temperature but will stay the same phase (solid, liquid, or gas) the whole time • Also known as specific heat calculations using a calorimetry 2) At a Phase Change – • These will have a constant temperature but will change phase (ex: solid to liquid) (We will worry about this one in the next video. Calorimetry • Measuring heat. • Use a calorimeter. • Two kinds – Coffee cup calorimeter and – Bomb calorimeter (Not used on AP test) © 2009, Prentice-Hall, Inc. Coffee Cup Calorimetry • One can indirectly measure the H for the system by measuring the heat change for the water in the calorimeter. • Heat =q = m x c x T • A coffee cup calorimeter measures H. H = qreaction /# moles • An insulated cup, full of water. • The specific heat of water is 4.18 J/gºC © 2009, Prentice-Hall, Inc. Specific Heat Definition: The amount of heat required to raise the temperature of one gram of substance by one degree Celsius. 5 4 6 5 7 4 3 8 3 2 9 2 1 11 6 7 8 9 1 10 Calculations Involving Specific Heat ( Remember: Phase stays the same the entire time. Ex. always a liquid.) q = mcT q = Heat lost or gained m =mass c = Specific Heat Capacity T = Temperature change Units for specific heat (c) are: Joules (gram) (Celsius) Examples within a phase Example 1: 5.83g of water was heated from 25.0ºC to 79.0ºC. The specific heat of water = 4.18 J/gºC How much energy was required to heat this sample? Given q= m= c= ΔT = q = m · c · ΔT Example 2: A block of ice was heated from -30.0ºC to -15.0ºC. It required 181J of heat for this to occur. The specific heat of ice = 2.06 J/gºC What was the mass of the block of ice? q = m · c · ΔT Example 3: A silver block (c = 0.235 J/gºC) weighing 10.5g was at 81ºC. It was then dropped into a container of hot liquid, and it absorbed 250.J of heat. What was the final temperature of the silver block? q = m · c · ΔT How do you calculate if it’s a solution? Same equation q = mcT q = Joules of heat m = total mass in calorimeter (solutes +solvent) (If a solution, use m = D * V to obtain the mass) Water has a density of 1.00 g/mL. We will assume the density of the solution is near that of water. (Write that statement on AP test!) c = specific heat (for water, 4.18 J/g-K) T = Tf - Ti A student performs an experiment to determine the molar enthalpy of solution of urea, H2NCONH2. The student places 91.95 g of water at 25°C into a coffee-cup calorimeter and immerses a thermometer in the water. After 50 s, the student adds 5.13 g of solid urea, also at 25°C, to the water and measures the temperature of the solution as the urea dissolves. A plot of the temperature data is shown in the graph below. (a) Determine the change in temperature of the solution that results from the dissolution of the urea. (b) According to the data, is the dissolution of urea in water an endothermic process or an exothermic process? Justify your answer. (c)Assume that the specific heat capacity of the calorimeter is negligible and that the specific heat capacity of the solution of urea and water is 4.2 J g-1 °C-1 throughout the experiment. (i) Calculate the heat of dissolution of the urea in joules. (ii) Calculate the molar enthalpy of solution, H © 2009, Prentice-Hall, Inc. References http://www.sciencegeek.net/Chemistry/Powerpoints2.shtml I modified the original PPT to fit our needs in AP Chemistry needs. Our textbook: Brown, Lemay et all. AP edition chemistry, 13th edition, 2015