U3 S3 L1 Calorimetry
advertisement

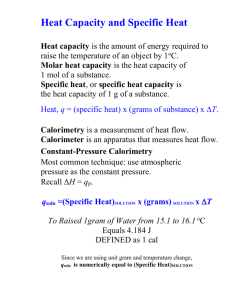
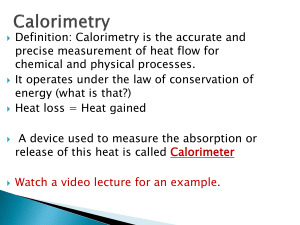
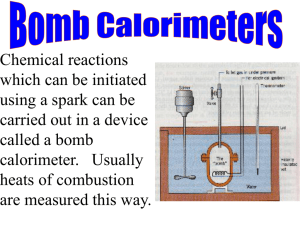
U3 S3 L1 Calorimetry Textbook Readings MHR •pages 660-661: The Technology of Heat Measurement •pages 665-667: Using a Calorimeter to Determine the Enthalpy of a Reaction •pages 673-674: Bomb Calorimetry Textbook Practice Items MHR •page 667: items 5, 6 and 7 •page 675: items 8, 9 and 10 •page 676: items 1, 2, *3 and *7 •page 704: item *10 •page 707: items 17, 18 and 21 Upon completion of this lesson, you should be able to: • perform calculations based on data collected from simple calorimetry experiments involving aqueous species to determine the molar heat of reaction • perform calculations based on data collected from bomb calorimetry experiments to show how energy changes associated with chemical changes can be used to determine reference data like molar heats of combustion and heat capacity • Calorimetry is the process of determining the energy change (q) associated with a physical or chemical change. qqsys = - qsurr • There are two basic types of calorimetry: 1. Simple (constant pressure) -Surrounding is the water in the calorimeter. q = mcwater∆T where Cwater=4.19 J/g•C 2. Bomb (constant volume) calorimetry - Surrounding is the calorimeter and its contents In these case the calorimeter contsnt ( specific heat of the calorimeter) is known. q = Ccal∆T where Ccal = ___kJ/C (ie: no mass) 4 Assumptions of Calorimetry: 1. The calorimeter is an isolated system, in that neither energy nor matter is lost or gained to the outside environment. 2. The first law of thermodynamic holds true. That is, energy is transferred from the system to the surroundings (vice versa). 3. Energy absorbed or released by the calorimeter itself is considered negligible. 4. Solutions involved in the chemical reactions are consider to be dilute and thus would have the density and specific heat capacity of pure water. 1 A volume of 50.0 mL of 0.50 M hydrochloric acid at 22.5°C was mixed with 50.0 mL of 0.50 M sodium hydroxide solution also at 22.5°C in a simple calorimeter. The highest temperature reached after mixing was 26.0°C. Calculate the molar heat of reaction of sodium hydroxide. 2 A 1.23 g sample of ethyne, C2H2, undergoes complete combustion in an oxygen bomb calorimeter resulting in a temperature increase of 9.50°C. The heat capacity of the calorimeter is 6.49 kJ/°C. Calculate the molar heat of combustion for ethyne. 4 In order to obtain calibration data for a bomb calorimeter, three 2.50 g samples of methanol were burned. The average temperature change of 4.23°C was recorded. The molar heat of combustion of benzene is -726 kJ/mol. Calculate the heat capacity of the bomb calorimeter