A 0
advertisement

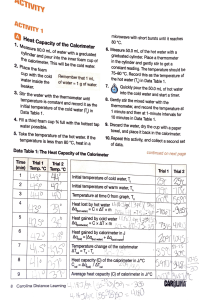
A 0.50 g sample of NH4NO3(s) is dissolved in 35.0 g of water in a Styrofoam cup. The temperature of the solution drops from 22.70C to 21.60C. i) ii) Is the process exothermic or endothermic? What is the heat of solution of ammonium nitrate, expressed in kJ/mol? Given the thermochemical equations: 2B(s) + 3/2 O2(g) → B2O3(s) B2H6(g) + 3O2(g) → B2O3(s) + 3H2O(g) H2(g) + ½ O2(g) → H2O(g) ∆H = -1273 kJ ∆H = -2035 kJ ∆H = -242 kJ Calculate the ∆H for the reaction: 2B(s) + 3H2(g) → B2H6(g) Calculate the pH of a 2.5 x 10-3 mol/L NaOH solution. Coffee cup calorimeters usually consist of Styrofoam cups with covers, thermometers, and stirrers. This apparatus absorbs a certain amount of heat during calorimetry experiments. Corrections for the heat absorbed by the coffee cup calorimeter can be carried out by using heat capacity. The heat capacity of a calorimeter is defined as the number of joules required to increase the temperature of the calorimeter by 1oC, measured in J/oC. A calorimeter at 20.0oC contains 25.00 g of water at 20.0oC. When 75.00g of NaOH is added to the calorimeter, the highest observed temperature is 34.4oC. Assuming no heat is lost from the calorimeter, what is the molar enthalpy of this reaction?