CHAPTER 18 KETONES AND ALDEHYDES
advertisement

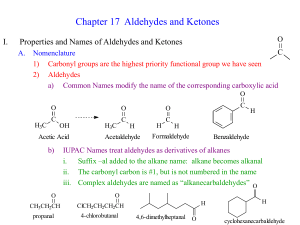
CHAPTER 18 KETONES AND ALDEHYDES PROBLEMS 1,3,4,6,7-11,12C 15,17,19,20,24,27A,C,31,33,34A,B,D, 35A,C,39A-E,45A,B,51A-D,61,65,66 CARBONYL COMPOUNDS O CONTAIN A C=O GROUP IMPORTANT MEMBERS O R O H R ALDEHYDE R KETONE CHAPTER 18 O O R OH CARBOXYLIC ACID CHAPTER 20 R O OR ESTER R O NH2 AMIDE CHAPTER 21 R Cl ACID CHLORIDE STRUCTURE OF THE CARBONYL GROUP DIPOLE MOMENT E+ C O H :NUC BASE: CHAPTER 22 NOTE: NUC ATTACK OCCURS AT PI ORBITAL NOT BACKSIDE OF C=O NOMENCLATURE IUPAC SYSTEM KETONES: ALKANONE, MUST NUMBER TO GIVE CARBONYL LOWEST NUMBER 1 2 3 4 5 6 O 5,5-DIMETHYL-3-HEXANONE (5,5-DIMETHYLHEXAN-3ONE) O O OH 4-HYDROXY-2-BUTANONE BUT O OH 3-OXOBUTANOIC ACID ALDEHYDES - IUPAC ALDEHYDE CARBON IS NUMBERED “ONE” 6 4 5 2 3 1 O 3-ISOPROPYLHEXANAL H O H FORMYLCYCLPENTANE 6 4 3 2 5 1 O O H 3-ISOPROPYL-5-OXOHEXANAL ALDEHYDES COMMON NAMES STEM PLUS ALDEHYDE R-CHO R-CO2H HCHO HCO2H FORMALDEHYDE FORMICA - ANTS CH3CHO ACETALDEHYDE CH3COOH ACETUM - SOUR CH3CH2CHO PROPIONALDEHYDE PROTOS PION - FIRST FAT PROPIONIC ACID CH3CH2CH2CO2H BUTRYM - BUTTER CH3CH2CH2CH2CH2CHO CAPROALDEHYDE ACETIC ACID CH3CH2CO2H CH3CH2CH2CHO BUTYRALDEHYDE FORMIC ACID BUTYRIC ACID CH3CH2CH2CH2CH2CO2H CAPER - GOAT CAPROIC ACID Ketones common names RADICAL SYSTEM NAME RADICALS IN ALPHA ORDER + KETONE O Me Methyl phenyl ketone STEM CONVENTION - ALKYL METHYL KETONES NAME STEM OF ALKYL GROUP ACCORDING tTO THE PREVIOUS SLIDE FOLLOWED BY PHENONE ACETOPHENONE O Write the structure for butyrophenone CH3CH2CH2 Ph SPECTROSCOPY OF ALDEHYDES AND KETONES 1. IR READ PAGE 780 2. NMR Read pp 780-782 MASS SPECTRA H3C ALPHA CLEAVAGE O H3C CH3CH2 + O H2 C H3C CH3 H2 C O CH3 + CH3 O TWO ACYLIUM IONS ARE ABOUT THE SAME H2 C CH3 O THEREFORE ETHYL RADICAL IS MORE STABLE THAN METHYL RADICAL STABILITY CONTROLS FRAGMENTATION McLAFFERTY REARRANGEMENT CHARACTERISTICS A GAMMA HYDROGEN IN THE PARENT CATION-RADICAL IS TRANSFERRED TO CARBONYL OXYGEN ATOM INTRAMOLECULARLY CLEAVAGE OF ALPHA-BETA CARBON (WITH RESPECT TO C=O OCCURS A STABLE ALKENE IS LOST AND A NEW RESONANCE STABILIZED CATION RADICAL(ENOL) IS FORMED NOTE- THE PARENT CATION, STABLE ALKENE LOST, AND THE NEW STABILIZED CATION RADICAL HAVE EVEN MOLECULAR MASSES EXAMPLE O a H3C C H2 H2 C b g CH2 OH H H3C CH2 even mw Even mw + CH2=CH2 g b Even mw MECHANISM OF McLAFFERTY REARRANGEMENT g O H3C C C H2 H CH2 CH2 OH H3C C + H2C CH2 CH2 THERE IS A NICE SUMMARY ON BOTTOM OF PAGE 784. LOOK IT OVER. REVIEW OF SYNTHESIS OF KETONES AND ALDEHYDES OXIDATION OF ALCOHOLS - LAST SEMESTER OZONOLYSIS OF ALKENES - LAST SEMESTER FRIEDEL ACEYLATION AND GATTERMAN KOCH WILL BE COVERED ON NEXT TEST HYDRATION OF ALKYNES - LAST SEMESTER HYDROBORATION AND OXIDATION - LAST SEMESTER 1,3-DITHIANE METHOD PREPARATION OF 1,3-DITHIANES O H HS-CH2CH2CH2SH H H+ S S HH Dream up a mechanism for this reaction. WHY HIGH ACIDITY OF 1,3DITHIANES? Old explanation S S H H S-S n-BuLi S H RECALL A-B has bonding mo and non-bonding mo neg charge A B Thus C S=C Vacant d-orbitals Modern explanation C S C Synthetic Methodology 1. Deprotonation of 1,3-dithiane with n-BuLi n-BuLi S S H S S H H Li 2. Alkylation or dithane anion (SN2) SN2 H S S S S + R-X Li H R R must be methyl or primary R can not be Ar, CH2=CH 3. Hydrolysis of alkylated dithiane. S H S R H+ / HgCl2 O H R Preparation of Ketones via 1,3dithianes INSTEAD OF STOPPING AT MONO-ALKYLATION STEP, ONE CAN REPEAT PROCESS USING THE REMAINING ACIDIC HYDROGEN ATOM S H S R R'X S R' S R O H+ HgCl2 R' R UMPOLUNG OF C=O O H S -S R electrophilic carbonyl H nucleophilic "masked" carbonyl "acyl anion equivalent" Retrosynthetic analysis Prepare 1-phenyl-2-pentanone From O H HSCH2CH2CH2SH H H S H S n-BuLi PhH2C n-BuLi S H S PhCH2B CH3CH2CH2Br PhH2C S CH2CH2CH3 S hydrolysis Ph O