File - AP CHEMISTRY
advertisement

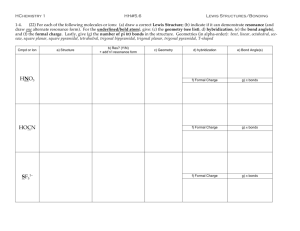
COVALENT BONDING Lewis Dot Diagram Valence Bond Theory Electrons in a covalent bond reside in a region that is the overlap of individual atomic orbitals. For example, the covalent bond in molecular hydrogen can be thought of as the overlap of two hydrogen 1s orbitals. Problem with VB Theory What is the predicted bond H-O-H angle in water? HYBRID ORBITALS To account for the shape (and resulting behavior) of molecules, chemists have devised hybrid orbitals. If you recall, s, p, d, and f orbitals exist in atoms. However, when atoms form covalent bonds, some of these orbitals “merge” or “fuse” to form hybrid orbitals. They have undergone hybridization. http://www.youtube.com/watch?v=SJdllffWUqg Carbon Atom in CH4 The four orbitals (2s, 2px, 2py, and 2pz) merge to form four sp3 orbitals of equal energy. VSEPR THEORY Lewis dot diagrams gives us a good ideas about which atoms are connected in a molecule. However, because it is a two dimensional representation of a three dimensional molecule, it has limitations. It does not give us molecular geometry, which is the spatial arrangement of the atoms. The Valence Shell Electron Pair Repulsion (VSEPR) model, is used to explain molecular geometry. VSEPR Theory states that electron pairs (shared or lone) tend to arrange themselves around an atom in such a way that repulsion between pairs is minimized. In other words, electron groups (domains) spread out from each other as much as possible. sp3 Tetrahedron or tetrahedral Bond angle between any two sp3 orbital is 109.5 degrees. Common examples: CH4, NH3, H2O Lone Pairs Effect on Bond Angles 109.5º 107º 104.5º Why would a lone pair occupy more space than a shared pair? 3 sp hybridization The four sp3 orbital repel each other as much as possible and form the “tetrahedral” shape. sp3 Shape of Molecules Lone pairs play a vital role in electron repulsion. However, lone pairs are "invisible" as far as the geometry of the atom is concerned. Only bonded atoms are considered when naming the shape of the molecule. http://www.youtube.com/watch?v=oDotFuloZTg&feature=related Shapes associated with 3 sp Tetrahedron Bent Trigonal pyramid Not Quite the Octet Rule sp2 hybridization Shapes associated with Trigonal (triangular) planar Bent 2 sp The unchanged porbital is capable of making a pi bond. Results in the trigonal planar arrangement. Bond angle is 120 degrees. Common examples: BF3, CH2O Even Less than Not Quite the Octet Rule sp hybridization Beyond the Octet Rule Elements with n values equal to or greater than 3, can have more than 8 electrons around it. 3 sp d hybridization Bond angles Associated with sp3d Shapes associated with Trigonal bipyramidal 3 sp d Linear T-shaped See-saw 3 2 sp d hybridization Shapes associated with Octahedral 3 2 sp d Linear Square planar T-shaped Square pyramidal Ethyne (Acetylene) – C2H2 The 2s and one of the 2p orbital merge to form the sp hybrid orbitals – 180 degree bond angle. The two remain 2p orbitals from each carbon atom merge together to create the double and triple bond. Multiple Bonds: Sigma vs Pi Bonds Single bonds (the first bonds that form) are always sigma (σ)π. The second or third bonds that form in double or triple bonds are always pi (π). Bond Length becomes shorter with each pi bond. Bond Strength increases with each pi bond. The molecule becomes more rigid with each pi bond. Ethene C2H4 What is the hybridization of each carbon atom? What is the bond angle? Impact of Multiple Bonds on Bond Angles 122º 116º 122º 122º 122º 116º Why would multiple bonds occupy more space than a single bond? Ethane – sp3 Ethene – sp2 Ethyne – sp REVIEW http://cost.georgiasouthern.edu/chemistry/general/molecule/quiz/frame4b.htm NITRATE ION Draw the Lewis Dot Structure of the NITRATE ION (NO3-1). If the above Lewis structure for nitrate were correct, the nitrate ion would have one bond that is shorter and stronger than the other two. UPON CLOSER EXAMINATION… Laboratory analyses show all three of the bonds in the nitrate ion to be the same strength and the same length. The behavior of the bonds suggests they are longer than double bonds and shorter than single bonds. They are also stronger than single bonds but not as strong as double bonds. HOW DO WE EXPLAIN THIS PHENOMENON? RESONANCE The double bond can occur between any oxygen atom and the central nitrogen atom. Each of these structures is called a resonance structure NITRATE ION The nitrate ion is not really changing from one resonance structure to another as previously thought. The ion behaves as if it were a blend of the three resonance structures. OTHER RESONANCE STRUCTURE Carbonate ion (CO3-2) Thiocyanate ion (SCN-) THE MOST FAMOUS RESONANCE STRUCUTRE BENZENE – C6H6 or or DELOCALIZED ELECTRONS CARBON NANOTUBES