lecture slides of chap10_updated
advertisement

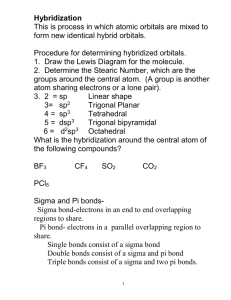
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Review Valence Shell Electron Pair Repulsion (VSEPR) Theory Valence Shell Electrons • the outer shell electrons of an atom, the ones involved in bonding • for a given elements, # of valence electrons = Group Number • C: Group 4A, 4 valence electrons O: Group 6A, 6 valence electrons Draw Lewis structure (pre-requisition) NH3 H N H H – Key: Electrons are all negatively charged – Action: electron pairs (bonding pairs & lone pairs ) around the central atom repel each other to keep themselves as far away as possible – Outcome: Maximum separation Minimum repulsion Symmetry (equal repulsion) – Applications: predict the electron pair geometry predict the bond angle predict the molecular geometry predict the hybridization of the central atom predict the polarity of the molecule – Electron pair geometry : the arrangement of all electron pairs (bonding pairs & lone pairs ) surrounding the central atom of the molecule. – Molecular geometry: the arrangement of only bonding pairs surrounding the central atom of the molecule VSEPR Class AB2 # of bonding pairs 2 # of lone pairs 0 Total # of electron pairs Electron pair geometry Molecular Geometry linear linear 2 180o B A B B A B VSEPR Class # of bonding pairs # of lone pairs Total # of electron pairs Electron pair geometry Molecular Geometry trigonal planar trigonal planar B B AB3 3 0 3 120o A A B B B trigonal planar AB2E 2 1 B bent 3 A A B B B B VSEPR Class # of bonding pairs # of lone pairs Total # of electron pairs Electron pair geometry Molecular Geometry tetrahedral tetrahedral B AB4 4 0 4 A B B B tetrahedral AB3E 3 1 A 2 2 A B B B B trigonal pyramidal 4 B AB2E2 B 109.5o A B B B tetrahedral bent A A 4 B B B B B Class AB5 # of bonding pairs 5 Total # of # of lone pairs electron pairs 0 5 Electron pair geometry Molecular Geometry trigonal bipyramidal trigonal bipyramidal 120 B 90o AB4E 4 1 B B o B A B B A B B B B trigonal bipyramidal seesaw B B 5 B B A A B B AB3E2 AB2E3 3 2 B B trigonal bipyramidal T-shaped B B 5 B 2 3 5 A B A B B trigonal bipyramidal linear B B A A B B VSEPR Class AB6 # of bonding pairs 6 # of lone pairs 0 Total # of electron pairs 6 Electron pair geometry Molecular Geometry octahedral octahedral B B B 90o B A B B B B B square pyramidal B AB5E 5 1 6 B A B B octahedral AB4E2 4 2 6 B B B B octahedral B B A B A B B B B A B B square planar B B B A B Bond angle Periodic table Electron pair geometry # of valance shell electrons Lewis structure Molecular geometry Total # of electron pairs around central atom Hybridization of the central atom What is the electron pair geometry, bond angle, molecular geometry and hybridization of the central atom of NH3? electron pair geometry: tetrahedral H N H bond angle: 109.5o molecular geometry: trigonal pyramidal H hybridization: sp3 Repulsive force lone-pair vs. lone pair lone-pair vs. bonding bonding-pair vs. bonding > > repulsion pair repulsion pair repulsion Predicting Polarity of Molecules: Dipole Moments and Polar Molecules m=Qxr Q is the charge r is the distance between charges electron poor region 1 D = 3.36 x 10-30 C m H d+ • A measure of the polarity of a molecule • The dipole moment can be determined experimentally • Measure in Debye units • Predict polarity by taking the vector sum of the bond dipoles electron rich region F d- 10.2 Molecules containing net dipole moments are called polar molecules. Otherwise they are called nonpolar molecules because they do not have net dipole moments. Diatomic molecules: Determined by the polarity of bond Polar molecules:HCl, CO,NO Nonpolar molecules: H2,F2,O2 Molecules with three or more atoms: determined by the polarity of the bond and the molecular geometry Dipole moment is a vector quantity, which has both magnitude and direction. • Symmetric molecules such as these are nonpolar because the bond dipoles cancel • All of the basic shapes are symmetric, or balanced, if all the domains and groups attached to them are identical C O C O Linear molecule Nodipolar moment O O bent molecule Net dipolar moment Which of the following molecules have a dipole moment? H2O, CO2, SO2, and CH4 O S dipole moment polar molecule dipole moment polar molecule H O C O no dipole moment nonpolar molecule H C H H no dipole moment nonpolar molecule 10.2 • Lewis structures and VSEPR do not tell us why electrons group into domains as they do • How atoms form covalent bonds in molecules requires an understanding of how orbitals interact Molecular Geometry & Hybridization Covalent Bonding Theories • Valence bond (VB) theory – Bonding is an overlap of atomic orbitals • Includes overlap of hybrid atomic orbitals • Molecular orbital (MO) theory – Bonding happens when molecular orbitals are formed 10.3 Hybridization – mixing of two or more atomic orbitals to form a new set of hybrid orbitals. 1. Mix at least 2 nonequivalent atomic orbitals (e.g. s and p). Hybrid orbitals have very different shape from original atomic orbitals. 2. Number of hybrid orbitals is equal to number of pure atomic orbitals used in the hybridization process. 3. Covalent bonds are formed by: a. Overlap of hybrid orbitals with atomic orbitals b. Overlap of hybrid orbitals with other hybrid orbitals 10.4 Predict the Hybridization of the Central Atom Total # of electron pairs Electron pair geometry Hybridization 2 linear sp BeCl2 3 trigonal planar sp2 BF3 4 tetrahedral sp3 5 trigonal bipyramidal sp3d PCl5 6 octahedral sp3d2 SF6 Examples CH4, NH3, H2O Total # of electron pairs = number of hybrid orbital (sum of the superscripts!) Formation of sp3 Hybrid Orbitals 10.4 Formation of sp Hybrid Orbitals 10.4 Hybridization in molecules containing double and triple bonds Two Kinds of covalent Bonds • Sigma bond: bonding density is along the internuclear axis – head to head overlap of two hybrid orbitals – head to head overlap of p + hybrid • any s character to the bond = sigma bond • Pi bond: bonding density is above and below the internuclear axis – Sideway overlap p + p 10.5 Sigma bond Pi bond Multiple bonds • Double bond: One sigma and one pi bond between the same atoms –Example: ethylene • Triple bond: One sigma and two pi bonds between the same atoms –Example: acetylene 10.5 Review of 3 Types of bonds: 1. Sigma bond e-density (overlap region) is along the internuclear axis 2. Pi bond e-density (overlap region) is above and below the plane of the internuclear axis. Relative reactivity of bonds: • Pi bonds are more reactive than sigma bonds • Less energy is needed to break a pi bond than a sigma bond 3. Delocalized Bonds • are not confined between two adjacent bonding atoms, but actually extend over three or more atoms. • less reactive than normal pi bonds • Example: benzene Summary: VB Theory framework of the molecule is determined by the arrangement of the sigma-bonds; Hybrid orbitals are used to form the sigma bonds and the lone pairs of electrons. Sigma (s) and Pi Bonds (p) Single bond 1 sigma bond 1 sigma bond and 1 pi bond Double bond Triple bond 1 sigma bond and 2 pi bonds How many s and p bonds are in the acetic acid (vinegar) molecule CH3COOH? H C O H C O H s bonds = 6 + 1 = 7 p bonds = 1 H 10.5 Valence bond theory O O Experiments show O2 is paramagnetic No unpaired e- Should be diamagnetic But experiment show that there are two unpaired electrons— paramagnetic. Molecular orbital theory – bonds are formed from interaction of atomic orbitals to form molecular orbitals. 10.6 Molecular Orbital (MO) Theory • Bonds are formed from interaction of atomic orbitals to form molecular orbitals. MO theory takes the view that a molecule is similar to an atom • The molecule has molecular orbitals that can be populated by electrons just like the atomic orbitals in atoms • # of MOs formed = # of atomic orbitals combined Energy levels of bonding and antibonding molecular orbitals in hydrogen (H2). A bonding molecular orbital has lower energy and greater stability than the atomic orbitals from which it was formed. An antibonding molecular orbital has higher energy and lower stability than the atomic orbitals from which it was formed. 10.6 Molecular Orbital (MO) Configurations -MOs follow the same filling rules as atomic orbitals 1. The number of molecular orbitals (MOs) formed is always equal to the number of atomic orbitals combined. 2. The more stable the bonding MO, the less stable the corresponding antibonding MO. 3. The filling of MOs proceeds from low to high energies. 4. Each MO can accommodate up to two electrons. 5. Use Hund’s rule when adding electrons to MOs of the same energy.(Electrons spread out as much as possible, with spins unpaired, over orbitals that have the same energy) 6. The number of electrons in the MOs is equal to the sum of all the electrons on the bonding atoms. 10.7 1 bond order = 2 bond order ½ ( Number of electrons in bonding MOs 1 - ½ Number of electrons in antibonding MOs ) 0 10.7