Biological Macromolecules – Polymers
advertisement

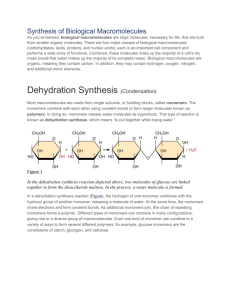
Biological Macromolecules – Polymers (The Importance of Carbon) Honors Biology Monkemeier Carbon Carbon can bond with itself to form chains, branched chains and rings. Carbon Skeletons When carbon bonds with itself to form the chains, branched chains and rings, the structures are known as carbon skeletons. Carbon skeletons provide the “backbone” for biological molecules. Carbon skeletons are formed during photosynthesis. Hydrocarbons Hydrocarbons are molecules composed of just carbon and hydrogen. The carbon – hydrogen bonds are HIGH in ENERGY. Other Elements Important to Biology and Functional groups. Oxygen, Hydrogen, Nitrogen, Phosphorus, Sulfur. These elements are part of functional groups. Functional groups are groups of atoms covalently bonded to each other and when attached to a “carbon skeleton” or molecule, the molecule gains their chemical and physical properties. Functional Groups Biological Molecules Biological Macromolecules Some molecules in biology are extremely large and are made by putting together smaller subunits. Biological Macromolecules are also known as POLYMERS. Poly = many and mer = pieces. Starch, Cellulose, DNA, Enzymes are all examples of biological macromolecules a.k.a. polymers. Building Biological Macromolecules The chemical reaction that attaches the “mers” or subunits together to form biological macromolecules is known as dehydration synthesis a.k.a. condensation. Dehydration Synthesis Dehydration Synthesis How is water involved? Why is it called dehydration synthesis? Examine the diagram to see. Hydrolysis a.k.a. Decomposition The chemical reaction that breaks apart biological macromolecules into their subunits is known as hydrolysis or decomposition. Hydrolysis a.k.a. Decomposition Hydrolysis (Decomposition) Why is it called hydrolysis? Hydro refers to water – why? Lysis means split – how does this relate?