Intermolecular Forces: Types, Strength & Effects
advertisement

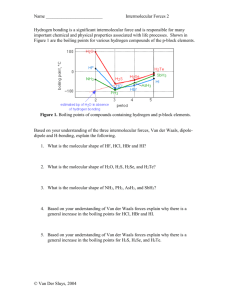
Chapter 3.5 Pages 54 - 59 Learning outcomes State the three types of intermolecular force. State what is needed for hydrogen bonding to occur. Describe how van der Waals and dipole-dipole forces arise. Describe how van der Waals forces affect temperature. Explain why NH3, H2O and HF have higher boiling temperatures than might be expected. Specification ref: 3.1.3 Practical Complete the worksheet to investigate the properties of the various substances Intermolecular forces Molecules and separate atoms are attracted to each other by weaker intermolecular forces. (inter means between). If intermolecular forces are strong enough, the molecules can be held closely enough together and can form liquids (e.g. bromine) or solids (e.g. iodine) at room temperature Types of intermolecular force There are three types of intermolecular force: van der Waals forces Acts between all atoms and molecules Dipole – dipole forces Acts only between certain types of molecules Hydrogen bonding Acts only between certain types of molecule Weakest Strongest Dipole – dipole forces Polar molecules = permanent dipoles δ+ of one molecule attracts the δ– of a different polar molecule to form a permanent dipole-dipole force. For example HCl Permanent dipole–dipole forces If molecules contain bonds with a permanent dipole, the molecules may align so there is electrostatic attraction between the opposite charges on neighbouring molecules. Permanent dipole– dipole forces (dotted lines) occur in hydrogen chloride (HCl) gas. The permanent dipole–dipole forces are approximately one hundredth the strength of a covalent bond. Complete the starter activity Check your answers! In molecules with more than one polar bond, the effects of each bond may cancel out, leaving a molecule that is non-polar (with no dipole moment). E.g. carbon dioxide is a linear molecule and the dipoles cancel. Both are equal and acting in the opposite direction Tetrachloromethane is tetrahedral and the dipoles cancel. Dichloromethane, the dipoles do not cancel because of the shape of the molecule Identifying polar molecules van der Waals’ forces Or... Induced dipole-dipole interactions... Exists between all molecules whether polar or non-polar Weak intermolecular attractions between very small, temporary dipoles in neighbouring molecules Van der Waals forces Strength of van der Waals forces 200 The strength of van der Waals forces increases as molecular size increases. This is illustrated by the boiling points of group 7 elements. boiling point (°C) 150 100 50 0 -50 -100 -150 -200 F2 Cl2 Br2 I2 element Atomic radius increases down the group, so the outer electrons become further from the nucleus. They are attracted less strongly by the nucleus and so temporary dipoles are easier to induce. Strength of van der Waals forces The points of contact between molecules also affects the strength of van der Waals forces. butane (C4H10) boiling point = 272 K 2-methylpropane (C4H10) boiling point = 261 K Straight chain alkanes can pack closer together than branched alkanes, creating more points of contact between molecules. This results in stronger van der Waals forces. Boiling points of alkanes van der Waals van der Waals forces act between all atoms or molecules at all times They are in addition to any other intermolecular forces The dipole is caused by the changing position of the electron cloud, so the more electrons there are, the larger the instantaneous dipole. The size of the van der Waals force increases with the number of electrons present. Boiling points Can you explain why the boiling points of the noble gases increases? Noble gas Boiling point/˚C Number of electrons He -269 2 Ne -246 10 Ar -186 18 Kr -153 36 Xe -108 54 Rn -62 86 Why does ice form such ccol shapes? https://www.youtube.com/watch?v=UukRgqzk-KE Hydrogen bonding https://www.youtube.com/watch?v=fWaSutDUpl4 What is hydrogen bonding? When hydrogen bonds to nitrogen, oxygen or fluorine, a larger dipole occurs than in other polar bonds. This is because these atoms are highly electronegative due to their high nuclear charge and small size. When these atoms bond to hydrogen, electrons are withdrawn from the H atom, making it slightly positive. The H atom is very small so the positive charge is more concentrated, making it easier to link with other molecules. Hydrogen bonds are therefore particularly strong examples of permanent dipole– dipole forces. Hydrogen bonding In molecules with OH or NH groups, a lone pair of electrons on nitrogen or oxygen is attracted to the slight positive charge on the hydrogen on a neighbouring molecule. hydrogen bond lone pair Hydrogen bonding makes the melting and boiling points of water higher than might be expected. It also means that alcohols have much higher boiling points than alkanes of a similar size. Hydrogen bonding and boiling points The boiling point of hydrogen fluoride is much higher than that of other hydrogen halides, due to fluorine’s high electronegativity. boiling point (°C) Boiling points of the hydrogen halides 40 20 0 -20 -40 -60 -80 HF HCl HBr HI -100 HF HCl HBr The means that hydrogen bonding between molecules of hydrogen fluoride is much stronger than the permanent dipole–dipole forces between molecules of other hydrogen halides. More energy is therefore required to separate the molecules of hydrogen fluoride. HI Permanent dipole–dipole forces Intermolecular Snap In pairs, take a pack of 20 cards. Shuffle these and deal out into two piles face down. Turn over a card each at the same time. It is snap if consecutive molecules have the same type of intermolecular attraction. Say which type of intermolecular attraction is present between each molecule. When snap is called, take it in turns to explain how it arises for the actual molecules on each card. e.g. water and ammonia snap since both form hydrogen bonds Water and methane don’t snap because methane cannot hydrogen bond. After ten goes reshuffle the cards and repeat.