the Intermolecular Forces Worksheet
advertisement

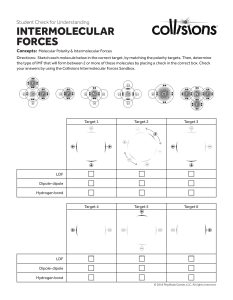
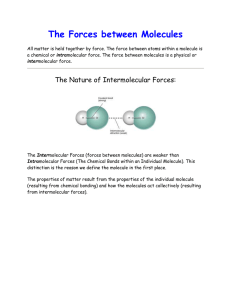
Name____________________________________ Using the information you find at the websites listed in Role 1 page of this webquest, answer the following questions: 1. The strength of intermolecular forces determines which of its macroscopic properties? 2. Draw and label the diagram which illustrates the difference between a intramolecular and intermolecular force 3. Intermolecular forces occur (within/between) molecules (circle correct answer) 4. What type of Intermolecular Forces (IMF) are the strongest? 5. Why is the Ion-Ion force between CaO stronger than that in KF? 6. In the molecule of ClF showing the bond dipole, why is the arrow pointing toward Fluorine? 7. ClF is a (polar / nonpolar) molecule (circle correct answer) 8. Draw the dipole-dipole IMF between the two ClF molecules 9. As the polarity of the molecule increases, the dipole-dipole (increases / decreases). 10. Why is the dipole-dipole force between F-Br molecules greater than that between F-Cl molecules? 11. Hydrogen bonding is a special kind of _____________________ that occurs when hydrogen is bonded to ________________, _______________ or ____________ 12. Draw the hydrogen bond between the two molecules of water. 13. Define London Dispersion Forces? 14. What is an Induced Dipole? 15. State the relationship between intermolecular forces and melting and boiling points. 16. List the different major types intermolecular forces. Rank them from the strongest to the weakest. 17. Complete practice question #1, then check your answer. 18. Complete practice question #2, then check your answer. 19. Complete practice question #3, then check your answer. 20. Complete practice question #4, then check your answer.