Condensed Phases and Intermolecular Forces
advertisement

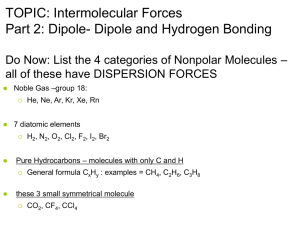
Condensed Phases and Intermolecular Forces Let’s look at particle diagrams of liquids & solids and compare them to particle diagrams of gases Describe & compare the relative positions and motions of particles in each of 3 phases: The Question: Why do some substances exist as gases, some as liquids, and some as solids at room temp? part of answer has to do with attractive forces between separate but neighboring molecules 2 broad categories of attractive forces come into play: 1. 2. INTRAmolecular forces of attraction INTERmolecular forces of attraction Forces INTERMOLECULAR INTRAMOLECULAR Dispersion Covalent Dipole-Dipole Ionic Hydrogen Bonding Metallic #1: Intramolecular Forces Intra means “within” Intramolecular attractive forces are chem bonding forces Definition: attractive forces that hold particles together in chemical bonds 3 types: ionic, covalent, or metallic bonds # 2: Intermolecular Forces (IMF) (aka: van der Waals forces) Inter means “between” or “among” Intermolecular forces: attractive forces between neighboring molecules Intermolecular forces are weaker than Intramolecular forces IMF: weaker than intramolecular (bonding) forces ≈ 5% to 15% of strength of intramolecular forces account strong weak for phase of matter at room temp IMF condensed phase (solid/liquid) IMF gas phase IMF determine phase of matter phase is determined by: “competition” IMF and KE between strength of If IMF are strong, substance will be solid or liquid at room temp strong attractive forces keep particles close together If IMF are weak, substance will be gas at room temp weak attractive forces allow particles to spread far apart & be free to move It’s all a balancing act! KE IMF [this substance = a gas at room temperature] Intermolecular Forces vs. Kinetic Energy IMF KE [this substance = a condensed phase (solid/liquid)] since T is measure of average KE, changing T can change phase changing T changes average KE of particles T change can allow change in phase 3 types of IMF: 1. Dispersion forces 2. Dipole-Dipole forces 3. Hydrogen bonds 1. Dispersion Forces: ● ● weakest IMF occur between non-polar molecules dispersion forces & non-polar molecules • instantaneous and momentary change • electron cloud will fluctuate • results from motion of electrons due to attractive forces Non-polar atoms/molecules non-polar means no poles • can’t tell one end of atom/molecule from other end • electrons are evenly distributed • charge is evenly distributed • atom/molecule: symmetrical Non-polar Atoms/Molecules: monatomic He, atoms: Ne, Ar, Kr, Xe, Rn 0 diatomic H2, elements: N2, O2, Cl2, F2, I2, Br2 0 small symmetrical molecules: CO2 , CX4 0 carbon-hydrogen CH4, C2H6, C3H8 molecules: Dispersion Forces and Size larger the electron cloud, the greater the fluctuations in charge can be strength of attractive dispersion forces ↑ with increrasing molecule size increasing strength of dispersion forces: Rn > Xe > Kr > Ar > Ne > He I2 > Br2 > Cl2 > F2 C8H18 > C5H12 > C3H8 > CH4 2. • • Dipole-Dipole forces: intermediate IMF occur between polar molecules What do you know about charge? Opposites Attract! this time, uneven distribution of electrons (charge) is permanent! examples: non-polar H2 CH4 polar HI CH3Cl Polar Molecules: geometry NOT symmetrical (asymmetrical) uneven electron distribution permanent separation of charge has poles: one end partly (-) and one end partly (+) neighboring molecules orient themselves according to their opposing attractive charges 3. • • • Hydrogen Bonding forces: strongest IMF subtype of dipole-dipole attractive forces attractive force occurs between H in one molecule and F, O or N in neighboring molecule H-F H-O or H-N Hydrogen Bonding Force H-O N-H 0 this attractive force occurs between molecules with FON!!! Hydrogen Bonding Force hydrogen bonding force is special subtype of dipole-dipole attractive forces F, O, and N are all small and electronegative strong electron attraction H has only 1 electron, so if being pulled away H proton is almost “naked” H end of molecule is always positive & F, O, or N end is always negative Strength of Hydrogen Bonding Force fluorine most electronegative element, so H-F bonds are most polar and exhibit strongest hydrogen bonding attractive forces H-F > H-O > H-N IMF vs. Physical Properties If strength of IMF then: If strength of IMF then: boiling point melting point heat of fusion heat of vaporization boiling point melting point heat of fusion heat of vaporization while: while: evaporation rate evaporation rate boiling point of N2 is 77 K (-196˚C) IMF are very, very weak forces (dispersion) Hydrogen bonding: • strongest IMF • influences physical props a great deal IMF vs. Temp IMF more important as temp is lowered low temperature = low evaporation rate high temperature = high evaporation rate Indicate type of IMF for each molecule: NH3 • Ar • N2 • HCl • HF • Ne • O2 • HBr • CH3NH2 • hydrogen bonding dispersion forces dispersion forces dipole-dipole forces hydrogen bonding dispersion dispersion dipole-dipole hydrogen bonding