Intermolecular Forces
advertisement
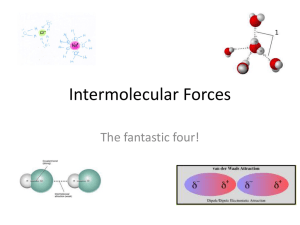
Intermolecular Forces Intramolecular Forces: ▫ The attractive forces between atoms and ions within a molecule ▫ Types: Ionic bond, covalent bonds (e.g. polar, nonpolar, double, triple) ▫ Relatively strong Intermolecular Forces: ▫ The attractive forces between molecules ▫ Weak (in comparison to intramolecular forces) ▫ Ex: much less energy to melt H2O (inter) than for it to decompose into H2 and O2 (intra) ▫ 3 types: Dipole-Dipole London Dispersion Hydrogen Bonding Intermolecular Forces & Physical Properties The strength of the intermolecular forces determines: • State • Melting point and boiling point • Surface tension • Hardness/texture • Solubility 1. Dipole-dipole • Forces of attraction between oppositely charged ends of polar molecules. • Relatively strong intermolecular force Dipole-dipole force London Dispersion • Attractive forces between all molecules, including nonpolar molecules • Result of temporary displacements of the electron cloud around atoms in a molecule (extremely short-lived dipoles) • Therefore, weaker than dipole-dipole Hydrogen Bonding • Very strong dipole-dipole force between the positive hydrogen atom of one H-bond molecule and highly electronegative atom of another molecule (N, O, F) Discussion/Recap: • Rank the intermolecular forces from weakest to strongest. • If one compound was gas at room temperature, and another was solid, what could you assume about their intermolecular forces? • How might intermolecular forces affect melting point? Practice • List the intermolecular forces in the following compounds: ▫ Cl2 ▫ H2O ▫ HCl Surface Tension • Molecules in a liquid are attracted by molecules on all sides • But, molecules at the surface are only attracted down or sideways. Practice! • Penny Activity • p. 85 # 6 • P. 88 # 10, 11