Chapter 5 Study Guide
advertisement

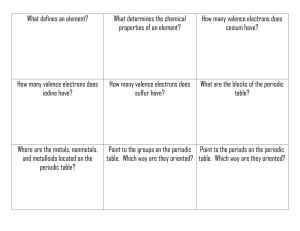
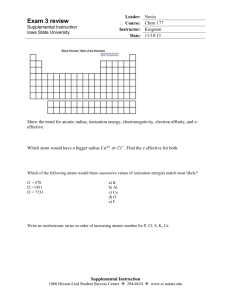
Technical Chemistry Chapter 5 Review Name Date Block DIRECTIONS: Using what you have learned about the periodic table and the elements, answer the following questions. Use your notes, text, worksheets, and periodic table as resources to help in answering these questions. 1. Discuss the following scientists. Be sure to include any important discoveries, experiments, theories, ideas, or laws that they are responsible for. a. JW Dobereiner b. Stanislao Cannizzaro c. JAR Newlands d. Dmitri Mendeleev e. Lothar Meyer f. Henry Moseley 2. What is the Periodic Law? Who developed this law? Why is it important? 3. Write the electron configurations for the following elements: a. Cs b. Ag c. Si d. Ga e. I f. Kr g. Zr h. Mg 4. Discuss the PERIODIC TRENDS: a. Atomic Radius b. Ionic Radius c. Ionization Energy d. Electronegativity e. Electron Affinity 5. Which has a larger atomic radius: Aluminum or Silicon 6. Which has a smaller atomic radius: Strontium or Rubidium 7. Which has a larger ionic radius: Fluorine atom or Fluorine ion 8. Which has a smaller ionic radius: Calcium atom or Calcium ion 9. Which has a larger 1st ionization energy: Lithium or Neon 10. Which has a larger 2nd ionization energy: Phosphorus or Sulfur 11. Which has a higher electronegativity: Chlorine or Iodine 12. Which has a lower electrongativity: Magnesium or Barium 13. Which ionization energy for Gallium is the highest: 1st, 2nd, 3rd, or 4th 14. Name an alkali metal 15. Name an alkaline earth metal 16. Name a metalloid 17. Name a transition metal 18. Name a halogen 19. Name a noble gas 20. Name an element in the lanthanide series 21. Name an element in the actinide series 22. Discuss the stability of Noble Gases 23. What are main group elements? 24. Determine the period, block, group, and identity of the elements ending in the following electron configurations. a. [Ar]4s23d8 b. [Kr]5s24d105p2 c. [Xe]6s24f145d5 d. [Rn]7s2 e. [Ne]3s23p5