Name: Date: Per: ______ #: ____ Period 6 Unit 2 Study Guide
advertisement

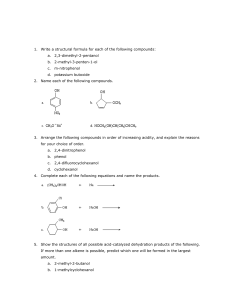
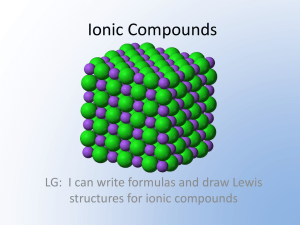
Name: _________________________________ Date: ______________ Per: ______ #: ____ Period 6 Unit 2 Study Guide Directions: Answer each of the following questions on a SEPARATE piece of paper. An answer key will be provided online but we will not go over the answers before the designated study day. If you would like help or have any questions outside of that time, please schedule time with me. 1. What doe BrINClHOF stand for and what is important about it? 2. Give the number of valence electrons for the following atoms? a. Cs e. Ge i. Ne b. Sr f. j. Ga c. Al g. O k. F d. Si h. Br l. As Xn 3. How do atoms become ions? 4. List 3 examples of cations. 5. List 3 examples of anions 6. What types of atoms typically form cations? 7. What types of atoms typically form anions? 8. Name the following ions. a. K+1 d. N-3 b. Mg+2 e. O-2 c. P-3 f. g. Al+3 Cl-1 9. Give the charges of the following ions a. Li d. Se g. Si b. Na e. I h. Al c. B f. i. S Ra 10. Where do we find valence electrons in an atom? 11. What is the difference between orbitals and energy levels? 12. Describe the octet rule and give an example of how an atom would follow this rule when bonding. 13. What are the 2 exceptions to the octet rule? 14. Draw the lewis dot structures for the following ions: a. K+1 c. P-3 e. O-2 b. Mg+2 d. N-3 f. Cl-1 g. Al+3 15. Describe how ionic compounds are formed. 16. What is another term for ionic compounds? 17. Draw the lewis dot structure for the following ionic compounds? a. KCl c. BeI2 b. MgCl2 d. Al2O3 18. List the properties of ionic compounds. 19. List the properties of covalent compounds. 20. List the properties of metallic compounds. 21. Determine if the following Lewis Dot Structures of COVALENT Compounds are correct. If not, explain WHY. (6pt) a. b. c. d. 22. List the properties of Acids. 23. List the properties of Bases. 24. Give 3 examples of acids. 25. Give 3 examples of bases. 26. Draw and label a pH scale. Include a label for acid, base, neutral, and numbers. 27. HCl has a pH of 1. How would we describe it using the pH scale? 28. Label this neutralization reaction: HCl + NaOH H2O + NaCl 29. For the following reaction. Circle the reactants, underline the products, and above the arrow, write what it represents. HCl + NaOH H2O + NaCl 30. List each of the types of chemical reactions. Describe each and give an example of a chemical equation for that reaction. 31. Define catalyst and enzyme.