PODs 61-64
advertisement

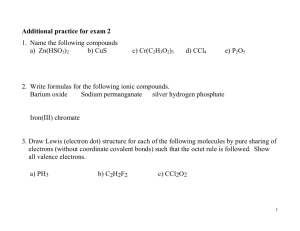
Chemistry PODs 61-64 POD #61 11/9/2015 Write your first and last name and today’s date in the upper right hand corner of your paper. Fold your paper into fourths. Be sure to number and date each POD. 1. Name the ions and ionic compounds given. a. H+ b. H- c. MgCl2 d. CaF2 2. Write formulas for the ions listed. a. Potassium chloride c. Aluminum chloride b. calcium bromide d. strontium fluoride POD #62 11/10/2015 Make sure that your PODs are numbered and dated. 1. Describe how to determine the number of valence electrons an element has by using its electron configuration. 2. Draw Lewis symbols for each element given. The atomic number is given to make it easier to locate the elements on your periodic table. a. Cs-55 b. Sn-50 c. Br-35 d. Al-13 Veteran’s Day No School POD #63 11/12/2015 Make sure that your PODs are numbered and dated. 1. What is the difference between a Lewis symbol and a Lewis structure? 2. Draw a Lewis structure to represent the formation of an ionic compound between Al and O. POD #64 11/13/2015 Make sure that your PODs are numbered and dated. 1. Name the compounds containing polyatomic ions. a. Mg(OH)2 b. K2Cr2O7 2. Write formulas for the compounds containing polyatomic ions. a. ammonium sulfate b. calcium iodate What’s On Our Quiz? • Quantum Theory and the Atom (p130-135) • Electron Configurations (p136-141) – Complete, noble gas, and configs for ions • • • • Monatomic Ions (p142-143) Simple Binary Ionic Compounds (p144-146) Valence electrons and Lewis Symbols (p152-154) Ionic Compound Formation and Lewis Structures (p155-156) • Types of chemical bonds (p157)