Chemical Formulas: The grammar of Chemistry
advertisement

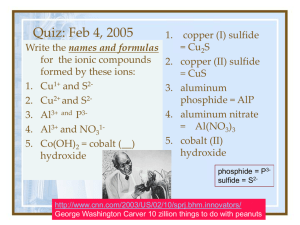
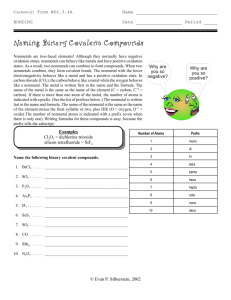
Quiz: Feb 3, 2005 1. calcium oxide = CaO Write the names and formulas for the ionic compounds 2. magnesium formed by these ions: sulfide = MgS 1. calcium and oxide 3. aluminum phosphide = AlP 2. magnesium and sulfide 4. aluminum 3. Al3+ and P3phosphate = 4. aluminum and phosphate AlPO4 5. magnesium and 5. magnesium phosphate phosphate= phosphide = P3Mg3(PO4)2 2sulfide = S “At age 58, Banneker began the study of astronomy and was soon predicting future solar and lunar eclipses. He compiled the ephemeris, or information table, for annual almanacs that were published for the years 1792 through 1797. "Benjamin Banneker's Almanac" was a top seller from Pennsylvania to Virginia and even into Kentucky.” http://www.progress.org/banneker/bb.html Balancing formulas from Worksheet Questions K+ 2O C O O 1. 2. 3. 4. 5. 6. 7. 8. 9. Na1+ K1+ Mg2+ Be2+ Sr2+ Cu1+ Zn2+ Ca2+ NH41+ F1CO32Cl1OH1S2S2I1PO43I1- NaF K2CO3 MgCl2 Balancing formulas from Worksheet Questions H K+ O Be 1. 2. 3. 4. 5. 6. 7. 8. 9. Na1+ K1+ Mg2+ Be2+ Sr2+ Cu1+ Zn2+ Ca2+ NH41+ F1CO32Cl1OH1S2S2I1PO43I1- NaF K2CO3 MgCl2 Be(OH)2 SrS Cu2S ZnI2 3O O P O O Ca2+ Ca2+ 3O O P O O 1. 2. 3. 4. 5. 6. 7. 8. 9. Na1+ K1+ Mg2+ Be2+ Sr2+ Cu1+ Zn2+ Ca2+ NH41+ F1CO32Cl1OH1S2S2I1PO43I1- NaF K2CO3 MgCl2 Be(OH)2 SrS Cu2S ZnI2 Ca3(PO4)2 NH4I 1+ 2+ 2- Oxidation number 1- • The charge on a monatomic ion is called the oxidation number. • An ion with more than one atom, has a different oxidation number on each atom, so the sum of the oxidation numbers equals the charge of the ion. • Nonmetals often have more than one oxidation state (number). • Transition metals may have more than one oxidation state (number). • Table 7.3 lists some common oxidation numbers . To name covalent compounds • Covalent compounds are composed of two or more nonmetals which share electrons. (Some metalloids are covalently bonded as well). • USE PREFIXES mono = 1 di = 2 tri = 3 tetra = 4 penta = 5 hexa = 6 hepta = 7 octa = 8 mono = 1 di = 2 tri = 3 tetra = 4 • CO2 • CO • P2O5 penta = 5 hexa = 6 hepta = 7 octa = 8 • carbon dioxide • carbon monoxide • diphosphorous pentoxide Try SF6 http://misterguch.brinkster.net/covalentcompounds.html click above for more information about covalent compounds Naming covalent compounds sulfur hexafluoride prefix of less electronegative atom (n ≠ 1), prefix of second atom– ending ide 1. 2. 3. 4. 5. 6. 7. 8. NaF K2CO3 MgCl2 Be(OH)2 SrS Cu2S ZnI2 Ca3(PO4) 2 9. NH4I 10. Mn(NO3 )3 11. FePO4 12. CoCO3 Answers. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. sodium fluoride potassium carbonate magnesium chloride beryllium hydroxide strontium sulfide copper (I) sulfide zinc iodide calcium phosphate ammonium iodide manganese (III) nitrate iron (III) phosphate cobalt (II) carbonate Writing Formulas potassium fluoride K+ and F- KF ammonium sulfate (NH4)2SO4 NH4+ and SO42- magnesium iodide Mg I2 copper (II) sulfite …. CuSO 3 Mg2+ and I 1Cu2+ and SO32- O 3O P O • Aluminum phosphate O 1C N AlPO4 Ag1+ • lead (II) nitrite Pb(NO3)2 • cobalt (II) selenide Al3+ O O H Pb2+ O N O 1- CoSe • silver cyanide AgCN • copper II bicarbonate Cu(HCO3)2 1- C Cu2+ O O 1- C H O O Naming covalent compounds • antimony tribromide • hexaboron (mono)silicide • chlorine dioxide SbBr3 • iodine pentafluoride I F5 • …. more examples B6Si ClO2 Writing Formulas for covalent compounds • P4S5 • O2 • tetraphosphorous pentasulfide • oxygen • SeF6 • selenium hexafluoride • Si2Br6 • disilicon hexabromide • SCl4 • … • sulfur tetra chloride CH4 methane, is an exception because it is an ORGANIC compound. Naming Organic Compounds meth = 1 eth = 2 prop = 3 but = 4 pent = 5 hex = 6 hept = 7 oct = 8 non = 9 … • Organic compounds have one or more carbons, surrounded with hydrogens. • They may have double or triple bonds, and may include oxygen, or other atoms. • They are named by counting the “carbon backbone” and applying a prefix • “Functional Groups” provide the rest of the name. Naming Organic Compounds meth = 1 eth = 2 prop = 3 but = 4 pent = 5 hex = 6 hept = 7 oct = 8 non = 9 … • Butane = 4 carbons • CH3CH2CH2CH3 • Heptane = 7 carbons • CH3CH2CH2CH2CH2CH2CH3 • Octane = 8 carbons • CH3CH2CH2CH2CH2CH2CH2CH3 13 c page 178 6 hydrogen atoms 3 carbon atoms 13 f page 178 6 carbon atoms 12 hydrogen atoms http://jchemed.chem.wisc.edu/JCESoft/CCA/CCA5/MAIN/1ORGANIC/ORG02/TRAM02/E/NOMOVIE/MISC.HTM Molecular and Empirical Formulas. • Molecular: adj. Pertaining to, consisting of, caused by, or existing between molecules. • Empirical: adj. 1. Relying upon or derived from observation or experiment. 2. Guided by practical experience and not theory, especially in medicine. • Formula: n 1. an established form of words for use in a ceremony or procedure. 3. Chemistry: a. a symbolic representation of the composition or of the composition and structure of a chemical compound. b. The chemical compound so represented. c. A prescription in exact proportion: recipe. Molecular and Empirical Formulas • C6H12O6 • H2O2 CH2O HO Coefficients: How many sets of a particular compound/element • 2 C6H12 Coefficients: How many sets of a particular compound/element • 5 C6H12