Oxygenation and Acid-Base Balance - Vanderbilt University Medical
advertisement

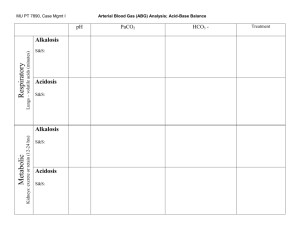
Interpretation: Compensated and Uncompensated Blood Gas Analysis James Barnett, RN, MSN Clinical Educator – Neuroscience PCC Vanderbilt University Medical Center May 2007 Compensatory Mechanisms Compensation is the body’s way of restoring a normal blood pH Remember: Acid + Base Neutrality Compensation DOES NOT treat the root of the problem – the reason for the acid-base imbalance is STILL THERE!!! Compensatory Mechanisms The body has three means to try to compensate for an acid-base imbalance Chemical Respiratory Renal Chemical Compensation Chemicals within the blood act within seconds to correct respiratory or metabolic imbalances Used up quickly – not effective long-term Chemical buffers in the blood include Bicarbonate Phosphate Proteins Respiratory Compensation Used to compensate for metabolic imbalances only Chemoreceptors respond to changes in H+ concentrations alters respiratory rate and depth Remember CO2 is an acid Respiratory Compensation Respiratory Rate will… Increase when blood H+ is increased (acidic pH) CO2 is “blown off ” Amount of acid in blood is decreased Decrease when H+ is decreased (alkaline pH) CO2 is retained Amount of acid in blood is increased Respiratory Compensation This means Metabolic acidosis causes an increase in rate and depth of ventilation as the body attempts to get rid of acid (CO2) Metabolic alkalosis causes a decrease in rate and depth of ventilation as the body attempts to retain acid (CO2) Renal Compensation Used to compensate for respiratory imbalances Remember: HCO3- is a base Kidneys respond to changes in blood pH Excrete H+ and retain HCO3- when acidemia is present (1:1 ratio) Retain H+ and excrete HCO3- when alkalemia is present (1:1 ratio) Renal Compensation This means A respiratory acidosis will make the kidneys excrete acid (H+) and retain base (HCO3-) A respiratory alkalosis will make the kidneys excrete base (HCO3-) and retain acid (H+) Renal Compensation This is the slowest compensation May take hours to days Most powerful method of compensation Ineffective in patients with renal failure Note on Compensation The body is very smart and will not overcompensate for an imbalance Degrees of Compensation An acid-base imbalance will be compensated for in one of three ways Uncompensated Partially compensated Fully compensated Degrees of Compensation Uncompensated Body has made no attempt to correct the acid-base imbalance Partially compensated Body is attempting to correct the imbalance Blood pH remains abnormal in spite of the attempt Degrees of Compensation Fully compensated The body is correcting the imbalance Blood pH is normal Other blood gas values remain abnormal until the root cause is treated and corrected Uncompensated Acid-Base Imbalance Uncompensated Imbalance pH abnormal Either PaCO2 OR HCO3- abnormal All other values normal If PaCO2 is abnormal Problem is respiratory If HCO3- is abnormal Problem is metabolic Uncompensated Imbalance Uncompensated respiratory acidosis Uncompensated respiratory alkalosis pH PaCO2 HCO3- pH PaCO2 HCO3- < 7.35 > 45 WNL > 7.45 < 35 WNL Remember that CO2 is an acid and that the more of it there is the worse is the acidemia. Notice that with uncompensated respiratory, the HCO3 is normal – this is because the body has not began to compensate for the alterations in CO2 Uncompensated Imbalance Uncompensated metabolic acidosis Uncompensated metabolic alkalosis pH PaCO2 HCO3- pH PaCO2 HCO3- < 7.35 WNL < 22 > 7.45 WNL > 26 Remember that HCO3 is a base and that the more of it there is the more alkalotic you will be. Notice that in the case of uncompensated metabolic the PaCO2 is normal indicating that the body has not began to compensate. Partially Compensated Imbalances Occur when compensation mechanisms are activated, but have not had sufficient time to normalize the blood pH NOTE: Some people say that there is no such thing as “partially” compensated – it is kind of like being “a little pregnant” – but it is indicative of a part of the process called compensation Partially Compensated Imbalances pH is abnormal Both PaCO2 and HCO3- are abnormal in the same direction (increased or decreased from normal) If PaCO2 is high (↑ acid), HCO3- will also be high (↑ alkaline) to neutralize the environment If PaCO2 is low (↓ acid), HCO3- will also be low (↓ alkaline) to neutralize the environment Partially Compensated Imbalances Partially Compensated Respiratory Acidosis Partially Compensated Respiratory Alkalosis pH PaCO2 HCO3- pH PaCO2 HCO3- < 7.35 > 45 > 26 > 7.45 < 35 < 22 In the case of Partially Compensated Resp Acidosis, the pH is low, indicating an acid environment…when you look at the PaCO2, it too is acidic, which is how you know that you have a respiratory acidosis. With the HCO3 being high, you can deduce that the body is raising its base to counteract the acid represented by the pH; therefore, partially compensated respiratory acidosis. Partially Compensated Imbalances Partially Compensated Metabolic Acidosis Partially Compensated Metabolic Alkalosis pH PaCO2 HCO3- pH PaCO2 HCO3- < 7.35 < 35 < 22 > 7.45 > 45 > 26 With partially compensated metabolic acidosis, you notice first that the pH is low (acidosis). Ask yourself, which number is representative of an acid condition. In this case it is the low base (HCO3), so you know you have a metabolic acidosis. You know it is partially compensated because the PaCO2 is low indicating that CO2 (an acid) is being lost from the body to correct for the low pH. Compensated Imbalances Occur when compensatory mechanisms have been able to fully normalize blood pH Compensatory Mechanisms Both PaCO2 and HCO3- are abnormal, but in the same direction If PaCO2 is high (↑ acid), HCO3- will also be high (↑ alkaline) If PaCO2 is low (↓ acid), HCO3- will also be low (↓alkaline) Compensated Imbalances Compensated Respiratory Acidosis Compensated Respiratory Alkalosis pH pH PaCO2 HCO3- PaCO2 HCO3- WNL but closer to 7.35 > 45 > 26 WNL but closer to 7.45 < 35 < 22 In compensated respiratory acidosis, the pH tends to range between 7.35 and 7.39 – still acidic, But in the normal pH range. When you look at the PaCO2, you notice that it is high (acidic), but The HCO3 is also high, indicating that the body has compensated and normalized the low pH. Compensated Imbalances Compensated Metabolic Acidosis Compensated Metabolic Alkalosis pH pH PaCO2 HCO3- PaCO2 HCO3- WNL but closer to 7.35 < 35 < 22 WNL but closer to 7.45 > 45 > 26 Mixed Imbalances Occur when patient has both metabolic and respiratory disorders that cause an acid-base imbalance Examples: Diabetic KetoAcidosis (metabolic acidosis) with decreased respiratory drive (respiratory acidosis) Severe vomiting (metabolic alkalosis) with high fever (respiratory alkalosis) Mixed Imbalances pH will be normal PaCO2 and HCO3- will be abnormal PaCO2 will be high with low HCO3- (both tend toward acid side) PaCO2 will be low with high HCO3- (both tend toward base side) Mixed Imbalances Mixed acidosis Mixed alkalosis pH PaCO2 HCO3- pH PaCO2 HCO3- < 7.35 > 45 < 22 > 7.45 < 35 > 26 Notice with the mixed acidosis that you have an acidic pH (less than 7.35, with other Parameters indicating an acid environment. High PaCO2 (too much acid). Low HCO3 (too little base – an acidic environment). This is classic mixed acidosis. Finished You have finished this in-service. Please go to the next in-service titled: Effects of Acid Base on Oxygenation