Chapter 20 - Wikispaces
advertisement

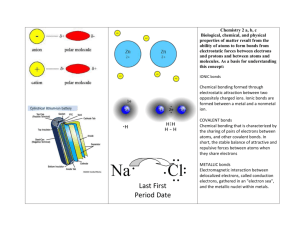
Chapter 20 Chemical Bonds Section 1 Stability in Bonding Reading Check Questions Pg. 603 Tells what elements are present in a compound and the exact number of the atoms of each element in a unit of that compound Caption Questions Fig 2 pg. 603 Table salt is a stable, white solid. Sodium is very reactive with water, silver in color, soft, and a metal. Chlorine is a poisonous, greenish-yellow gas. Fig 3 pg. 604 An atom fewer than 4 electrons in the outer level tends to give up electrons, while those with more than 4 tend to gain. Self Check 1. Sodium, as an element, is a solid metal. Chlorine is a gas. When two combine to form sodium chloride, they form a solid compound. 2. The compound ratio is made up of one barium ion and two floride ions. Also, since the compound is made up of a metal and a nonmetal, it is likely ionic. Self Check 3. Chemical Bonds 4. If an element has 8 electrons in outer electron energy level, it has a tendencey not to react. 5. Electrons are either gained, lost, or shared between atoms. 6. Hydrogen, carbon, and oxygen are in the compound. Each unit contains four hydrogen, two carbon, and two oxygen atoms. 7. 54. 86 % Section 2 Types of Bonds Reading Check Questions Pg. 609 The superscript written after the element’s symbol Pg. 614 A molecule that has a slightly negative end and a slightly positive end Caption Questions Fig 11 pg. 611 N2 Self Check 1. In ionic bonds, one atom accepts electrons from another. This happens only among elements having large differences in their attractions for electrons. 2. In ionic bonds, one atom accepts an electron from another atom. In covalent bonds, electrons are shared, sometimes unequally Self Check 3. Covalent bonding produces molecules 4. Ionic: Ca – O and K – O, because K and Ca are metals far from O on the periodic table; covalent: S – O because S and O are nonmetals close to eachother on the table. 6. The charge on Al is +3 and the charge on O is (2 (+3)) + (3 (-2)) = 0 Section 3 Writing Formulas and Naming Compounds Reading Check Questions Pg. 621 Hepta- Caption Questions Fig 18 pg. 620 Element -ide name CaSO4 Oxygen Oxide phosphorus Phosphide Nitrogen Nitride Sulfur Sulfide Caption Questions Name Oxidation Number Copper (I) 1+ Copper(II) 2+ Iron (II) 2+ Iron (III) 3+ Chromium (II) +2 # of Atoms 1 2 3 4 5 6 7 8 Prefix MonoDiTriTetraPentaHexaHeptaOcta- Integrate History Pg. 617 CaO Applying Math Practice Problems pg. 617 Pb3P4 Fe2O3 Applying Science Pg. 618 1. Copper (II) oxide 2. Aluminum chloride Self Check 1. Kl; Mg(OH); Al2(SO4)3; Cl2O7