Chapter 6.1 - chamilton
advertisement

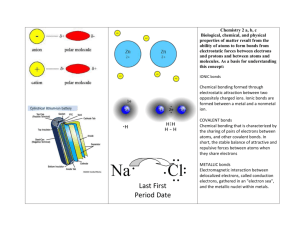
Chapter 6.1 Elements combine to form compounds Definition of Compound • A substance made up of atoms of two or more different elements. The Elements of a periodic table: • Can form millions of compounds • Atoms of different elements are held together by chemical bonds. Chemical Bonds • Can hold atoms together in large networks or small groups • Help determine the properties of a compound. The properties of a compound depend on: • which atoms the compound contains • how the atoms are arranged The properties of a compound are often very different from the properties of the elements that make them. • Calcium is a soft, silvery, metallic solid. • Chlorine is a greenishyellow gas that is extremely reactive and poisonous to humans. • Together they make Calcium Chloride which is a non-porous, white solid used to melt ice on streets Atoms Combine in Predictable Numbers A given compound always contains atoms of elements in a specific ratio. Definition of Chemical Formula: • An expression that shows the number and type of atoms joined in a compound. Definition of Subscript • A number written to the right of a chemical symbol and slightly below it in a chemical formula. • The subscript of 1 is never used. • Ex) CO2 Use the Chemical Formula chart on Page 172: • How many more hydrogen atoms does propane have than methane? • Why is the ratio of atoms in a chemical formula so important? Use the Chemical Formula chart on Page 172: • How many more hydrogen atoms does propane have than methane? 4 • Why is the ratio of atoms in a chemical formula so important? Different ratios of elements indicate different compounds The properties of compounds can be different even if they are made up of the same elements 6.1 Review Questions, pg. 173 Answer each question in complete sentences. Answers to 6.1 Review 1. In many cases, they are different 2. There are 45 total atoms in the formula; 12 carbon, 22 hydrogen, and 11 oxygen. 3. Atoms can combine in many different ratios. 4. All atoms are in a 1:1 ratio. 5. Compare how they react with other substances. 6. The ratios of the compounds are different. Ch. 6.2 Chemical bonds hold compounds together Chemical bonds between atoms involve electrons • When do chemical bonds form? – When valence electrons (electrons in the outermost shell) in the electron cloud around two atoms interact 1. Chemical bonds between atoms involve electrons • Atoms can transfer electrons (ionic bonds) • Atoms can share electrons (covalent bonds) • Chemical bonds give all materials their structure. 2. Atoms can transfer electrons. • When is a positive ion formed? A positive ion is formed when an atom loses electrons. • When is a negative ion formed? A negative ion is formed when an atom gains electrons An element’s location on the periodic table can give a clue as to the type of ions that atoms of that element will form • What type of ions do metals form? + ions (Group 1 loses 1 e-(electron),Group 2 loses 2 e-) • What type of ions do nonmetals form? - ions (Gr. 16 gains 2 e-, Gr. 17 gains 1 e-) • What group on the table does not normally form ions? Why? Noble Gases (group 18); because their outer energy level are full. Ionic Bonds • How are ionic bonds held together? by the force of attraction between positive and negative ions (transfer electrons) • Ionic compounds form between all nearby ions of opposite chare. These interactions make ionic compounds very stable and their crystals very strong. • Octet Rule – Rule of 8 8e- in outer shell = stable atom Ionic Bonds • Ionic bonds form between a metal (+ ion) and a nonmetal(ion). Ionic Bonds • Steps for naming a chemical compound: 1. Take the name of the positive metal element 2. Take the name of the negative nonmetal element 3. Combine the two names. 3. Atoms can share electrons. Nonmetal atoms usually form bonds by sharing electrons. Covalent Bonds • How are covalent bonds held together? By sharing electron pairs • How can you help yourself remember how covalent bonds are held together? Co = partner What does each line in the model stand for? • A shared pair of electrons What is a molecule? • A molecule is a group of atoms held together by covalent bonds. 4. Chemical bonds give all materials their structures. • What is responsible for many of the properties of the substances? The structure of their crystals and molecules that make it up Ionic Compounds • Have a regular crystal structure • Because of these rigid structures, when enough force is applied to the crystal it shatters rather than bends. Covalent Compounds • How are covalent compounds different from ionic compounds? Covalent bonds exist as individual molecules • How does molecular structure affect the properties of compounds? Ex. We detect scents because molecules fit into receptors in our nose drugs can work because molecules fit into specific receptors in the body. Polar Covalent Bond • The unequal sharing of electrons between two atoms that gives rise to negative and positive regions of electric charge. Ch. 6.2 Review Questions pg.182 1. What part of an atom is involved in bonding? 2. How are ionic bonds and covalent bonds different? 3. What kind of bond would you expect strontium and iodine to form? Why? Name the compound Ch. 6.2 Review Questions pg.182 1. What part of an atom is involved in bonding? Electrons in the cloud (valence e-) 2. How are ionic bonds and covalent bonds different? Ionic bonds transfer and are held together by electrical attraction, covalent bonds share electrons 3. What kind of bond would you expect strontium and iodine to form? Why? Name the compound Ionic bond – metal + nonmetal Strontium is in Gr. 2 and forms a + ion; Iodine is in Gr. 17 and forms a – ion. They have opposite charges and attract. Name: Strontium Iodide