study guide
advertisement

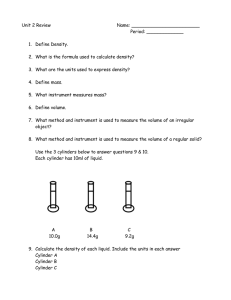
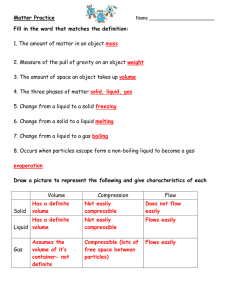
Corresponding page in notes Chemistry Semester 1 Study Guide 1. Chemistry is the study of ______________________________________________________ 2. What is the proper way to dispose of the following items: A) Chemicals: B) Used paper towels: C)Matches: Pg. 1-2 3. Identify the independent variable, dependent variable, constants, and control in the following experiment scenario: Megan wants to see if playing her favorite music to plants will affect their growth. She buys 20 tomato plants from Wal-Mart. She waters and fertilizes all 20 plants. Megan places 10 plants along a windowsill in a quiet room. She puts the other 10 plants along a window sill in the next room with continuously playing music. Both of these rooms are facing south and both are kept at a temperature of 72° Fahrenheit. Every day she equally waters the plants. She also records the growth of every plant. Pg5-6 Pg 4 In folder “What’s the Matter” 4. Convert the following: a) 100m= __________km c) 5.7cg= __________mg b) 56L= ___________mL d)17g= ___________kg 5. Identify what is being measured (length, volume or mass) based on the following units: Kilometers nanograms Grams cm3 milliliters millimeters 6. What is matter? 7. Circle the examples of matter: Electricity Heat Car Wind Water Sunlight Air Fear Hydrogen Pg 16 8. Fill in the information for the phases of matter. Phase Particle Movement Shape Volume Solid Liquid Gas Pg 10 9. What is the difference between intensive & extensive properties? 10. Circle the following properties that are extensive. Pg 10 Volume Hardness Length Density Flammability Boiling Point Reactivity Mass Produces CO2 when heated 11. Which of the following are examples of a chemical change? Pg 12 From scaven ger hunt (in folder) Explosion Freezing Gas being produced Melting Rusting Tearing Boiling Burning Spoiling 12. Fill in the information of the models of the atom as it has changed over time. Model 1st suggestion Solid Sphere Plum Pudding Rutherford Model Bohr (solar system) Model Year Who Important feature/Discovery Electron Cloud Model 13. Label the parts of the atom: Pg 19 & 24 Pg 1920 Pg 20 Pg 22 Pg 19 Nucleus Proton Neutron Electron Valence Electron 14. Fill in the information about the subatomic particles: Subatomic Particle Location Mass Proton Neutron Electron Charge 15. Match the key feature to the subatomic particle it belongs to. Subatomic Particle Key Feature A. Proton Responsible for chemical reactions B. Neutron Identifies the element C. Electron Added with protons, gives mass of atom 16. Label the parts for both ways of writing isotopes. Atomic Number Mass Element Name/Symbol Carbon-14 14 C 6 17. What information is given from the following sample square from the periodic table? (ex. Protons, neutrons, electrons, etc) 6 C 12.00 18. Name the groups of the periodic table & identify the number of valence electrons for each Main Group. Pg 32 & 24 Pg 25 & 26 19. Draw the Bohr for the following model and Lewis Dot Structure elements: Lithium Sodium Fluorine Chlorine Pg 27 20. Give the electron configuration and draw the orbital diagram (PreAP) for each of the following elements: Lithium Sodium Fluorine Chlorine Pg 3537 21. Define and describe (which direction increasing) each of the trends for the periodic table: Atomic Mass Atomic Size Reactivity Ionization Energy Electronegativity Shielding Effect Menu of activities/ video notes online 22. Describe what must take place for the following elements to become ions. What charge do they become and do they become cations or anions? Sodium Oxygen Phosphorus Gallium Pg 7-8 (?) 23. Calculate the density of an object that has a mass of 48g and a volume of 12mL. 24. What is the density of water? 25. What does the density of an object have to be in order to sink in water?