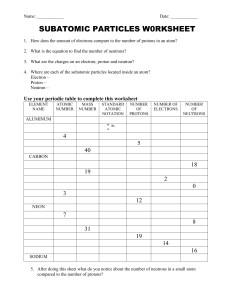
Name: _________________________ Chemistry Quiz 1-Retake 1. What is an atom? a. Smallest unit of an element that still retains properties of the element b. A neutron c. A subatomic particle d. A cell 2. Which of the following is a subatomic particle of the atom? a. Proton b. Neutron c. Electrons d. All of the above are included 3. Which subatomic particle determines the identity of the element? a. Proton b. Neutron c. Electron d. Molecule 4. Which subatomic particle is responsible for forming bonds and can cause electricity? a. Proton b. Neutron c. Electron d. Protein 5. What subatomic particle differs in an isotope? This particle adds mass to the isotope but does not have any charge a. Proton b. Neutron c. Electron d. Phase change 6. Fill in the following chart with the following words: Positive, negative, neutral, small (1/2000 amu), large (1 amu), inside the nucleus, outside the nucleus in a cloud, p, e-, n Proton Electron Neutron Charge Size Location Symbol 7. What is an isotope? ______________________________________________________________________ 8. Why does the atomic mass number tell us the number of protons and neutrons but NOT electrons? Name: _________________________ a. Electrons are so small that they do not contribute much mass b. Protons are so small that they do not contribute much mass c. Neutrons are so small that they do not contribute much mass 9. The atomic number of an element tells you the number of ___________ in an atom. a. Protons b. Neutrons c. Electrons d. Molecules 10. How many neutrons are in this element?: _______________________ 11. Boron (B) Bohr Model (Circles around the nucleus): Lewis DOT structure: 12. What is the electron configuration for: Sodium (Na) Name: _________________________ Lithium (Li): __________________________________ Boron (B): ____________________________________ Nitrogen (N): ___________________________________ 13. Calculate the average atomic mass of bromine. One isotope of bromine has an atomic mass of 78.92amu and a relative abundance of 50.69%. The other major isotope of bromine has an atomic mass of 80.92amu and a relative abundance of 49.31%.