Aldehydes and Ketones
advertisement

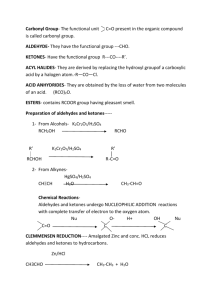
4.4 Aldehydes and Ketones 1 Aldehydes and Ketones O H R R C H O H C C C H H H C H R •Carbonyl functional group •Aldehyde: terminal carbon •Ketone: non terminal carbon 2 Aldehydes and Ketones Aldehydes are prepared by a controlled oxidation of the corresponding primary alcohol. The aldehyde is distilled off as it forms to prevent further oxidation. R R 2-- H C O H H Cr2O7 + H C O H 3 Aldehydes and Ketones Aldehydes are oxidised by acidified dichromate ions to the corresponding carboxylic acid. O O 2-- Cr2O7 R R C + C H H O H 4 Aldehydes and Ketones Ketones are prepared by the oxidation of secondary alcohols. No further oxidation occurs. R H H H C C C O H R Cr2O7 2-- R C C + H O H H H H C H H R 5 Aldehydes and Ketones When heated with Tollen’s reagent (ammoniacal silver nitrate), aldehydes are oxidised to carboxylate ions. RCHO RCOO– The silver diamine ion is reduced to metallic silver which can be deposited on the test tube giving a silver mirror. Ag(NH3)2+ Ag 6 Aldehydes and Ketones Ketones (and alcohols) do not oxidise with Tollen’s reagent. The silver mirror test is commonly used to identify between aldehydes and ketones. It can also be used to identify between an aldehyde and alcohol where dichromate ions would react with both. 7 Tetracycline (antibiotic) Vanillin 8 4.5 Carboxylic Acids 9 Carboxylic Acids O H R C H Carboxyl group C O H Carboxylic acids can be prepared by oxidation of primary alcohols or aldehydes with excess acidified dichromate solution. 10 Carboxylic Acids Carboxylic acids are weak acids If they are soluble in water then they will partially ionise to form hydronium and carboxylate ions. RCOOH + H2O H3O+ + RCOO– Equilibrium position favours reactants. 11 Carboxylic Acids Carboxylic acids react readily with bases such as: Hydroxides and Oxides (Products: Salt & Water) RCOOH + OH– RCOO– + H2O Carbonates (Products: Salt ,Water and CO2) 2RCOOH+CO32– 2RCOO– +H2O+CO2 Hydrogen carbonates (Products: Salt ,Water and CO2) RCOOH + HCO3– RCOO– + H2O+CO2 12 Carboxylic Acids Carboxylate salts of sodium and potassium are water soluble due to strong ion-dipole interactions which form between the negative carboxylate ion and the water molecules H O H R C H H O C O– H H O 13 Ion dipole interactions Strongest type of secondary interaction Occurs between a full positive or negative charge (ion) and a polar molecule Common between carboxylate ions and water or protonated amines and water 14 Carboxylic Acids Many drugs including (aspirin and other painkillers) contain a carboxyl group. These drugs are often mixed with solid sodium hydrogen carbonate which converts the acid to the water soluble carboxylate on mixing with water. This makes the drug easier to take and quicker acting that it would be in its insoluble form. 15 Citric Acid Salicylic Acid 16 4.6 Amines 17 Amines Based on ammonia H R C H H 1o (primary) amine N H H R 2o (secondary) amine C H N H H C H R 18 Amines R H C H R C H 3o (tertiary) amine N H H C H R Amines tend to have unpleasant odours, often fishy. 19 Amines Like ammonia, amines can act as a base accepting a proton from an acid NH3 + H+ → NH4+ RNH2 + H+ RNH3+ The product of this reaction is referred to as the protonated form of the amine The protonated form is soluble in water due to ion dipole interactions between the protonated amine ion and water 20 Amines Many drugs (eg. Anaesthetics) contain amine functional groups These drugs are insoluble in water and so would not be effective if administered as the amine By protonating these drugs they are able to dissolve in water within the body and so can act H O H H H H N+ C H H R 21 Nicotine Chlorphenamine (Antihistamine) Methyl Orange 22 4.7 Esters 23 Esters O H R C H Ester functional group C O H Acid C H R Alcohol Similar structure to carboxylic acids Hydrogen on carboxyl group replaced by alkyl group Fruity odour 24 Naming Esters Identify the alcohol carbon chain and the carbon chain from the acid. Name the alcohol part first followed by the acid H H H H O H C C C C H H H H H H C C C O H H H Ethyl pentanoate 25 Methyl Cinnamate (Strawberry) Aspirin (Acetyl Salicylic Acid) Oil of Wintergreen (Methyl Salicylic Acid) 26 Production of Esters Esters are formed by the reaction of a carboxylic acid and alcohol This reaction is called ESTERIFICATION but can also be referred to as a CONDENSATION reaction because water is one of the products. The reaction is slow at room temp and so the reaction mixture must be refluxed for an extended period and concentrated sulfuric acid is used as the catalyst This is an equilibrium reaction 27 Reflux 28 Production of Esters The yield of the ester can be increased by using excess of one of the reagents. (Usually the alcohol because it is cheaper) Large carbon chains (R groups) on the acid and alcohol decrease the yield of ester O H H C H H + C H O reflux H C H O O H H H2SO4 H C H C H C H O H + H O H 29 Production of Esters The ester can be separated from the mixture using a separating funnel The mixture is washed with sodium bicarbonate which ensures the acid dissolves in the water layer 30 Production of Esters The aqueous layer which contains any unreacted alcohol and the sodium carboxylate salt can be tapped off leaving the organic layer This layer is then distilled and the ester is collected at the appropriate tb 31 Distillation 32 Reactions of Esters If an ester is refluxed with aqueous acid or base, it undergoes hydrolysis In acidic conditions the products formed are a carboxylic acid and an alcohol. The reaction mixture is refluxed with sulfuric acid as the catalyst This is the reverse of esterification Acidic conditions R H C O O R + H O R H+ reflux R C O O + O H H 33 Reactions of Esters In alkaline conditions the products formed are the carboxylate ions and the alcohol The ester is refluxed with sodium hydroxide solution and the alcohol product can be separated from the sodium carboxylate salt by distillation The carboxylic acid will reform if a solution of strong acid is added to the sodium carboxylate salt. R Alkaline conditions R – O C O O R + R + C H O– O O H 34